We haven’t talked much in these pages about atmospheric pressure when it comes to characterizing exoplanets, but recent discussions of ‘super-Earths’ and thick, hydrogen/helium atmospheres have raised the issue. All but simultaneously came the news of a paper from Amit Misra (a University of Washington graduate student) and co-authors describing a new way of detecting atmospheric pressure on exoplanets. Misra’s simulations of Earth’s own atmospheric chemistry involved teasing out the signature of dimer molecules from light at various wavelengths. While a monomer is a molecule that may bind chemically to other molecules, a dimer is a chemical compound made up of two similar monomers bonded together.
Misra’s work is intriguing because the stability of water on a planet’s surface depends not just on temperature but pressure — the latter affects water’s boiling point and sublimation. Estimating surface pressure thus becomes an indicator for potential habitability. The problem is that making the call on pressure in a planetary atmosphere is tricky, involving the widths of individual absorption lines in spectral measurements. What Misra is proposing is to use the distinctive spectral features of dimers — in particular, oxygen dimers — that display their own rotational and vibrational modes that are distinguishable from their constituent O2 molecules.
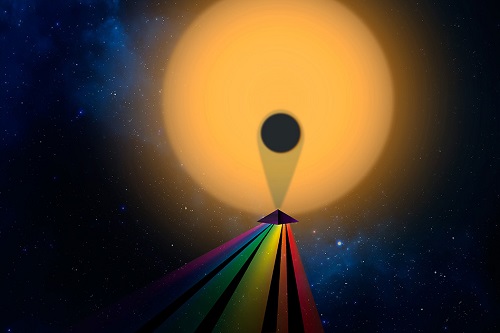
Image: Studying a planetary atmosphere by examining the transmission spectrum as the planet transits its star. Credit: Christine Naniloff/MIT, Julien De Wit.
Usefully, the distinctive patterns of dimers are sensitive to pressure, so that space-based observatories like the James Webb Space Telescope should be able to look for their particular absorption pattern, the presence of which, according to Misra, tells us that the planet has at least one-quarter to one-third the pressure of the Earth’s atmosphere. The researcher adds that because oxygen dimer molecules are more detectable than other markers of oxygen, they may play an important role in the detection of potential biosignatures:
“It’s tied to photosynthesis, and we have pretty good evidence that it’s hard to get a lot of oxygen in an atmosphere unless you have algae or plants that are producing it at a regular rate. So if we find a good target planet, and you could detect these dimer molecules — which might be possible within the next 10 to 15 years — that would not only tell you something about pressure, but actually tell you that there’s life on that planet.”
Working with Victoria Meadows (University of Washington), Mark Claire (University of St. Andrews) and Dave Crisp (JPL), Misra simulated both transit transmission spectra (when a planet transits the star and some of the star’s light moves through the atmosphere) and direct imaging spectra, where the planet is observed independently. The fact that dimer absorption features change more rapidly with pressure and density than those of monomers turns out to be most useful when studying the atmospheres of planets around M-class dwarfs, and the team’s work shows that JWST should be able to make such detections for an Earth analog planet orbiting an M-dwarf at a distance of 5 parsecs, allowing us to set a lower bound on pressure.
The paper is Misra et al., “Using Dimers to Measure Biosignatures and Atmospheric
Pressure for Terrestrial Exoplanets,” Astrobiology Vol. 14, No. 2 (2014), 67-86 (full text).



Would this have been able to detect the oxygen in Earth’s atmosphere in the Proterozoic Era, when it was only about 1%?
I forgot the link to the original published paper:
http://online.liebertpub.com/doi/pdfplus/10.1089/ast.2011.0733
And here in UniverseToday:
http://www.universetoday.com/109859/martian-meteorite-could-have-contained-ancient-water-and-life-nasa-paper-says/
What about abiogenic oxygen? There could be whole range of escape velocities and exospheric temperatures when hydrogen leaves into space, but oxygen remains in the process of water photodissociation, creating super-earths with very dense oxygen atmospheres from oceanworlds and mini-neptunes – some kind of http://arxiv.org/pdf/1310.2590.pdf , but with water on a planetary scale. If the water content in waterworld is substantial, the amount of oxygen that can be released could be much higher than the needed to completely oxidize all the solids in the mantle and core.
There could even be a mechanism of complete photodissociation of oceans in waterworlds. At tens and hundreds of kbars of pressure, solid oxygen is denser than high-pressure ice, so a layer of solid oxygen may form _under_ the water layer, keeping the water on the surface until it disappears completely. Guess the two inner planets of Kepler-11 system are oxygen “gas” giants!
Torque : a newly formed planet in the habitable zone wil probably start out with a thick atmosphere containing an excess quatity of hydrogen relative to oxygen . In the early stages this wil result in very high temperatures and a rapid loss of hydrogen . As the atmosphere gets thinner and less insolating , and the planet generally coools down , water becomes a stable liquid and the loss of hydrogen slows down to earth-like quantities . Inside a certain range of size and distance from the star , this might be a regulating mecanism which will result in waterworlds starting out without much free hydrogen OR free oxygen in their atmospheres ..
>>Inside a certain range of size and distance from the star , this might be a regulating mecanism which will result in waterworlds starting out without much free hydrogen OR free oxygen in their atmospheres ..
This may work for hydrogen inside some tight range of conditions, but closer to inner edge of HZ it would probably become more uncertain – as the moist greenhouse sets in, stratosphere become saturated with H2O and photodissociation accelerates wildly. Then it depends on exospheric temperature and escape velocity, if the latter is very high, both H and O atoms don’t escape, and we have a hot neptune. If it’s lower, only H escapes: since mean free velocity of H is four times higher than that of O, we can get a situation when m.f.V of H atoms is only four times lower than V(esc), and it escapes readily, but for O atoms it’s 16 times lower. That’s possibly the Venus case, but the oxygen quantity wasn’t enough there to properly oxidise the crust. (in the narrower range of masses and insolations, that may possibly apply to H and He atoms as well, creating massive He- and He-O-dominated atmospheres) But what if water constitutes 25% of a planet’s mass? or if the heavy waterworld is in the middle of a conventional HZ, but He content and thus the amospheric insulation is so high that temperatures never drop enough and moist greenhouse persists? We can get a 4 M(earths) and 1.6 R(earths) world with the earthlike insolation and plenty of atmospheric oxygen, but not distinguish if it was produced by photodissociation or photosynthesis…
Also this could mean we can get human-habitable but not inhabited worlds with breathable atmospheres (but some of that would make the voice squeaky :-) )
Consider that under oxydizing conditions the abiogenic organic molecules that are thought to have allowed life to form would not be stable, so life is much less likely to emerge on a world with abiogenic oxygen.