Finding the right conditions for life off the Earth justifiably drives many a researcher’s work, but nailing down just what might make the environment beneath an icy moon’s surface benign isn’t easy. The recent wave of speculation about Enceladus revolves around the discovery of phosphorus, a key ingredient for the kind of life we are familiar with. But Alex Tolley speculates in the essay below that we really don’t have a handle on what this discovery means. There’s a long way between ‘habitable’ and ‘inhabited,’ and many data points remain to be analyzed, most of which we have yet to collect. Can we gain the knowledge we need from a future Enceladus plume mission?
by Alex Tolley
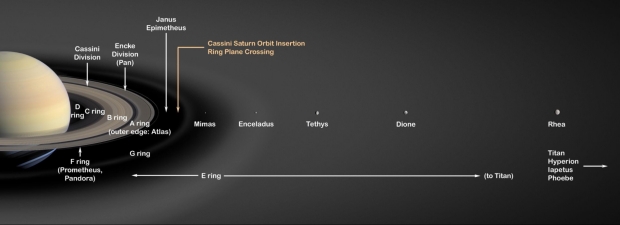
There has been abundant speculation about the possibility of life in the subsurface oceans of icy moons. Europa’s oceans with possible hydrothermal vents mimicking Earth’s abyssal oceans and the probable site of the origin of life, caught our attention now that Mars has no extant surface life. Arthur C Clarke had long suggested Europa as an inhabited moon in his novel 2010: Odyssey Two. (1982). While Europa’s hot vents are still speculative based on interpretations of the surface features of its icy crust, Saturn’s moon, Enceladus, showed visible aqueous plumes at the southern pole. These plumes ejected material that contributes to the E-Ring around Saturn as shown below.
While most searches for evidence for life focus on organic material, it has been noted that of the necessary elements for terrestrial life, Carbon, Hydrogen, Oxygen, Nitrogen, Sulfur, and Phosphorus (CHONSP), phosphorus is the least abundant cosmically. Phosphorus is a key component in terrestrial life, from energy management (ATP-ADP cycle) and information molecules DNA, and RNA, with their phosphorylated sugar backbones.
If phosphorus is absent, terrestrial biology cannot exist. Phosphorus is often the limiting factor for biomass on Earth, In freshwater environments phosphorus is the limiting nutrient [1]. Typically, algae require about 10x as much nitrogen as phosphorus. If the amount of available nitrogen is increased, the algae cannot use that extra nitrogen as the amount of available phosphorus now determines how large the algal population can grow. The biomass-to-phosphorus ratio is around 100:1. When phosphorus is the limiting nutrient, then the available phosphorus will limit the biomass of the local plants and therefore animals, regardless of the availability of other nutrients like nitrogen, and other factors such as the amount of sunlight, or water. Agriculture fertilizer runoff can cause algal blooms in aqueous environments and may result in dead zones as oxygen is depleted by respiration as phytoplankton blooms die or are consumed by bacteria.
While nitrogen can be fixed by bacteria from the atmosphere, phosphorus is derived from phosphate rocks, and rich sources of phosphorus for agriculture were historically gleaned from bird guano.
A recent paper in Nature about the detection of phosphorus in the grains from the E-ring by the Cassini probe’s Cosmic Dust Analyzer (CDA) suggested that phosphorus is very abundant. As these grains are probably sourced from Enceladus’ plumes, this implies that this moon’s subsurface ocean has high levels of dissolved phosphorus.
The authors of the paper have modeled, and experimentally confirmed the model, and make the claim that Enceladus’ ocean is very rich in phosphorus:
…phosphorus concentrations at least 100-fold higher in the moon’s plume-forming ocean waters than in Earth’s oceans.
around 100-fold greater than terrestrial phosphorus abundance levels. They show that the CDA spectrum [figure 1) is consistent with a solution of disodium phosphate (Na2HPO4) and trisodium phosphate (Na3PO4) (figure 2) The source of these salts on Enceladus is likely from the hot vents chemically releasing the material from the carbonaceous chondritic rocky core and the relatively alkaline ocean. Contrary to intuition, the greater CO2 concentrations in cold water with the hydroxyapatite-calcite and whitlockite-calcite buffer system maintain an alkaline solution that allows for the high phosphate abundance in the plume material that produces the grains in Saturn’s E-ring.
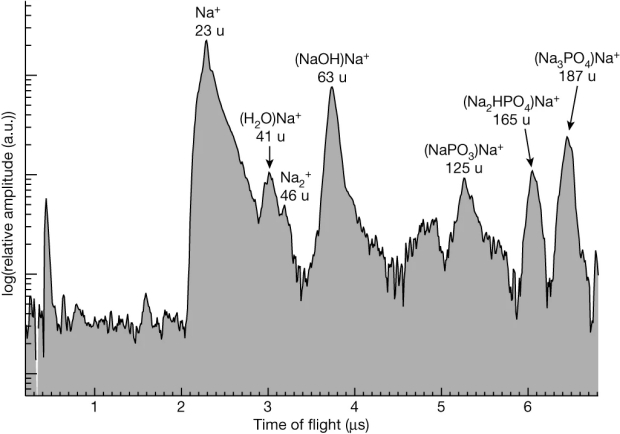
Figure 1. CDA cation spectrum co-added from nine baseline-corrected individual ice grain spectra. The mass lines signifying a high-salinity Type 3 spectrum are Na + (23 u) and (NaOH)Na + (63 u) with secondary Na-rich signatures of (H2O)Na + (41 u) and Na 2+ (46 u). Sodium phosphates are represented by phosphate-bearing Na-cluster cations, with (Na3 PO4)Na + (187 u) possessing the highest amplitude in each spectrum followed by (Na2HPO4 )Na + (165 u) and (NaPO3)Na + (125 u). The first two unlabelled peaks at the beginning of the spectrum are H + and C +, stemming from target contamination 3 (source nature paper). a.u., arbitrary units.
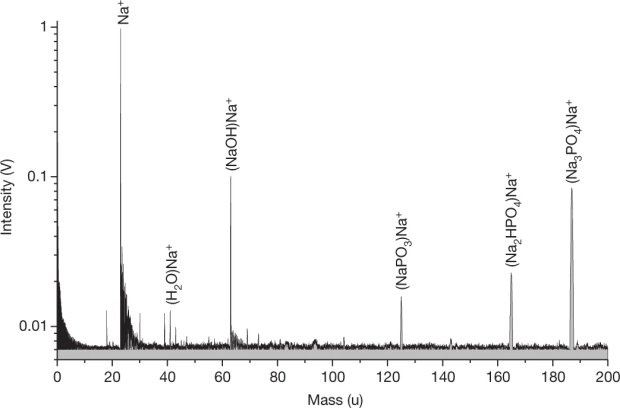
Figure 2. Spectrum from the LILBID analogue experiment reproducing the features in the CDA spectrum. An aqueous solution of 0.420 M Na2HPO4 and 0.038 M Na3PO4 was used. All major characteristics of the CDA spectrum of phosphate-rich grains (Fig. 1) are reproduced at the higher mass resolution of the laboratory mass spectrometer (roughly 700 m/?m). Note: this solution is not equivalent to the inferred ocean concentration. To derive the latter quantity, the concentration determined in these P-rich grains must be averaged over the entire dataset of salt-rich ice grains. (source Nature paper).
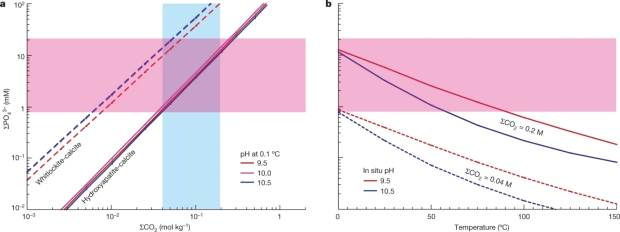
Fig. 3: Comparison of observed and calculated concentrations of ΣPO43– in fluids affected by water–rock reactions within Enceladus. a, Relation between ΣPO43– and ΣCO2 at a temperature of 0.1 °C for the hydroxyapatite-calcite buffer system (solid lines) and the whitlockite-calcite buffer system (dashed lines). Constraints on ΣCO2 obtained in previous studies are indicated by the blue shaded area. The area highlighted in pink represents the range of ΣPO43– constrained in this study from CDA data. b, Dependence of ΣPO43– on temperature for the hydroxyapatite-calcite buffer and different values of pH and ΣCO2. A similar diagram for the whitlockite-calcite buffer can be found in Extended Data Fig. 11.
The simple conclusion to draw from this is that phosphorus is very abundant in the Enceladan ocean and that any extant life could be very abundant too.
While the presence of phosphorus ensures that the necessary conditions of elements for habitability are present on Enceladus, it raises the question: “Does this imply Enceladus is also inhabited?”
On Earth, phosphorus is often, the limiting factor for local biomass. On Enceladus, if phosphorus was the limiting factor, then one would not expect it to be detected as inorganic phosphate, but rather in an organic form, bound with biomolecules.
But suppose Enceladus is inhabited, what might account for this finding?
1. Phosphorus is not limiting on Enceladus. Perhaps another element is limiting allowing phosphates to remain inorganic. In Earth’s oceans, where iron (Fe) can be the limiting factor, adding soluble Fe to ocean water can increase algal blooms for enhanced food production and possible CO2 sequestration. On Enceladus, the limiting factor might be another macro or micronutrient. [This may be an energy limitation as Enceladus does not have the high solar energy flux on Earth.]
2. Enceladan life may not use phosphorus. Some years ago Wolfe-Simon claimed that bacteria in Mono Lake used arsenic (As) as a phosphorus substitute. [2] This would have been a major discovery in the search for “shadow life” on Earth. However, it proved to be an experimental error. Arsenic is not a good substitute for phosphorus, especially for life already evolved using such a critical element, and as is well-known, arsenic is a poison for complex life.
3. The authors’ modeling assumptions are incorrect. Phosphorus exists in the Enceladan ocean, but it is mostly in organic form. The plume material is non-biological and is ejected before mixing in the ocean and being taken up by life. The authors may also have wildly overestimated the true abundance of phosphorus in the ocean.
Of these explanations allowing for Enceladus to be inhabited, all seem to be a stretch that life may be in the ocean despite the high inorganic phosphorus abundance. Enceladan biomass may be constrained by the energy derived from the moon’s geochemistry. On Earth, sunlight is the main source of energy maintaining the rich biosphere. In the abyssal darkness, life is very sparse, although it can huddle around the deep ocean’s hot vents.
However, if life is not extant, then the abundance of inorganic phosphorus salts is simply the result of chemical equilibria based on the composition of Enceladus rocky core and abundant frozen CO2 where it formed beyond the CO2 snow line.
While the popular press often conflate habitability with inhabited, the authors are careful to make no such claim, simply arguing that the presence of phosphorus completes the set of major elements required for life:
Regardless of these theoretical considerations, with the finding of phosphates the ocean of Enceladus is now known to satisfy what is generally considered to be the strictest requirement of habitability.
With this detection, it would seem Enceladus should be the highest priority candidate for a search for life in the outer solar system. Its plumes would likely contain evidence of life in the subsurface ocean and avoid the difficult task of drilling through many kilometers of ice crust to reach it. A mission to Enceladus with a suite of life-detecting instruments would be the best way to try to resolve whether life is extant on Enceladus.
The paper is Postberg, F., Sekine, Y., Klenner, F. et al. Detection of phosphates originating from Enceladus’s ocean. Nature 618, 489–493 (2023). https://doi.org/10.1038/s41586-023-05987-9
References
1. Smil, V (2000) Phosphorus in the Environment: Natural Flows and Human Interferences. Annual Review of Energy and the Environment Volume 25, 2000 Smil, pp 53-88
2. Wolfe-Simon F, et al (2010) “A Bacterium That Can Grow by Using Arsenic Instead of Phosphorus. SCIENCE 2 Dec 2010, Vol 332, Issue 6034 pp. 1163-1166
DOI:10.1126/science.1197258



One question that isn’t being addressed is how long has the Saturnian system been in its current configuration. Saturn’s moons, ring system and Saturn itself shows signs of a recent disruptive event. There may not have been enough time for life to have evolved on Enceladus.
Finding phosphorus on or near Europa – that would be far more exciting (and closer to us)
That’s a solid point. The recent interest in the age of the ring system is instructive.
It is a fair point, especially if the modeling suggests Enceladus is just around 100m years old. However, because of surface changes, there is no impact record to determine the age. While we find fossils indicating terrestrial life was extant 3.8 bn ya, we don’t know if life could have started earlier given the right conditions. So it is possible that even a very young Enceladus could have life from abiogenesis.
IF there is life on Enceladus, AND IF it is a very young world, then this would hugely increase the probability of life in the galaxy and potentially complex life or even intelligent, technological life. That is an enticing prospect that makes me want to find out whether there is life there or not. I am not optimistic about life in the subsurface oceans of any icy moon, but we won’t know if we don’t look. Unlike detecting biosignatures of exoplanets, the only near-term hope we have of studying the biology of ET life is if it is discovered in our system. A discovery would be very exciting, and worth the cost of searching, from my POV.
If the solar system outside of Earth is sterile, and there is no evidence of pre-biotic chemistry, then that gives our descendants the option to “green” the solar system if they choose to do so. If the galaxy appears sterile too, then bringing life to the galaxy may be a very important task for humanity.
Is anyone doing Miller-Urey experiments on a simulated Enceladus (or generic icy moon) environment? Or is information on nutrient and energy availability too limited as yet?
There have been many variations on the Miller-Urey experiment. The problem is that while amino acids are easy to generate – they appear wherever we look in space – the experiment does not get us any further. Different experiments can create short proteins (oligopeptides) from amino acids, but these also stop short of anything more. Unless we think the missing ingredient is just 0.1 to 1.0 bny, then what we need is a better understanding of abiogenesis.
As asteroids have amino acids, sometimes even chirally biased, an asteroid impacting Enceladus with its amino acid payload obviates the need for de novo synthesis in the subsurface ocean.
The Miller-Urey experiment is oversold in the media. We know it doesn’t replicate the origin of life, because polymerization of amino acids into proteins depends on a massive rRNA machine, the ribosome. Even the first ribosome must have taken a very long time to evolve, presumably in an “RNA World”. (Non-ribosomal protein synthesis does exist, but it isn’t plausible as a means by which proteins began)
The Miller-Urey experiment shows that (as in space) amino acids are compounds that fairly “easily” form in a CHON chemical soup. There are not other compounds so much more favorable on the basis of thermodynamic or kinetic considerations at room temperature that would deplete the component elements from the environment. At least, not if that environment includes a source of energy to shake things up. This tells us that several amino acids were plausibly available before life began, and could have taken part in the first metabolic pathways. But it doesn’t prove they did.
The first thing I thought of on hearing of the original article was the question of whether an ice-world’s rocky core and its heavier elements was locked away from the ocean by a layer of higher-order ices. Was that never a concern with Enceladus or does the article just not mention it?
The article assumes direct interaction between the rocky core and the ocean to generate the needed P as phosphate. It does not say whether the entire rocky core surface is in contact with the ocean, or whether just at the vents. But contact is needed for the P to be created to be found in the E-ring and by implication, in the subsurface ocean. The authors even acknowledge that the implied high P levels may be due to just local production at the hot vents and not indicative of the whole ocean.
If this moon does not have an ecosystem to preserve, the news may come to be of practical interest. They estimate the plumes contain 0.8 to 21 mM phosphate (24.8 to 651 milligrams per liter: probably more than most soft drinks) Enceladus is already a source of ice for terraforming, but now it comes with fertilizer? Gravity is less than 2% of Earth’s; escape velocity is 860 km/hr. Enceladus is tidally locked, so I imagine you could build a track on the ground there that is designed to slingshot large loads of this ice around Saturn (with some course correction) to land most places in the Solar system. When this falls from down from Saturn orbit to some crater on Moon or Mars or Mercury that is shaded at the moment of impact, I imagine you could end up with a pool of boiling water that would soon freeze over to become a central resource for a new space colony?
Not so different from the plot of Asimov’s “The Martian Way” where the Martian colonists used the ice in Saturn’s rings as a water supply for their rockets when denied access to earth’s Oceans.
Well… While many are pretty excited (and perhaps a touch overly exuberant!) about this phosphorous detection from Enceladus…
A really key central point implied by Alex Tolley above, is that if Enceladus was blooming with life and a vibrant biosphere, and if that life was chemically similar to Earthly life in some key ways, then…
You’d expect the phosphorous to be used up, and/or bound to organic molecules.
The fact that this does not appear to be the case, for now, kinda slaps down some of the initial excitement, and throws some cold (geyserly water!) right in my face, for me at least!
————————————
In the end:
This might very well mean that there’s nothing there to take advantage of the phosphorous, so it’s just simply going wasted and getting dissolved into an empty lifeless ocean.
But sure, of course: there still could remain the possibility of an alien biosphere in Enceladus’ oceans… all running on a much more alien chemistry than earthly life, that doesn’t like or need phosphorous, but… ya… again, this just isn’t the greatest sign in my books.
————————————
Couple that with some wondering if Europa’s oceans are hyper-super-salty (well beyond even the salt levels of the dead sea here on Earth–and remember: they don’t call it the “Dead Sea” for nothing!) and I’m suddenly rebounding, and swinging back like a pendulum, into a pessimistic-phase about discovering alien life in our own backyard solar system.
:(
————————————
ANOTHER NOTE about Europa’s oceans:
On yes, here on Earth we have halophiles that have evolved to withstand some very salty conditions! But 2 things to keep in mind about that:
1) That life started off in much nicer, far more pleasant and conducive conditions to life, and then from there GRADUALLY evolved to survive extreme salt. In other words: ancient humans gradually evolved to live outside of Africa, including the arctic, but it’s unlikely you’d find a creature like humans evolving in the arctic! They’d need an “encubator” like Africa first. Also they weren’t just plunged into the Arctic, again, they migrated and adapted gradually.
2) But even with Earthly halophiles, it is thought that there are some hyper-super salty conditions, that not even the strongest halophiles would be able to ever really adapt to, even gradually.
————————————
So if all of those assumptions are true…
Then again, I’m much more pessimistic about life at Europa or Enceladus unfortunately.
But who knows: maybe next month that personal pendulum will swing back again, and I’ll be envisioning the “near certainty” that there “MUST!” be life there. And hopefully there is.
The lack of phosphorus uptake seems pessimistic for Earth life, suggesting limits on energy or other elements. After all, we have deeply entrenched genetic, structural, and metabolic uses for phosphorus. Yet Earth life doesn’t use every element willy-nilly: we almost ignore silicon, sitting right beside phosphorus on the periodic table, even though it is one of the most common elements on the planet. Even phosphorus is almost only heard of in the context of phosphorus-oxygen bonds. There is some research to suggest that phosphonates with P-H bonds may be underappreciated, but we don’t need them for DNA, cell membranes, or energy storage.
To me, our dependence on phosphorus seems like a historical accident — almost an Achilles heel, given how it limits overall biomass and the degree to which “Gaia” can do useful things like take up CO2 out of the atmosphere. I think it dates back to the very beginning of life, when (in my favorite hypothesis) formaldehyde passing over hydroxylapatite was converted to ribose by the formose reaction, and soon created the first ribose phosphates ready to react with nitrogen compounds to form nucleotides. Converting these to sugars and fats and eventually cell membranes would come later. If life started some other way, I don’t think its core biology would be dependent on this uncommon element.
You are no different from the scientific community regarding pendulum swings about life elsewhere. Once it was assumed that intelligent life was everywhere. Lowell was a major proponent of intelligent life on Mars. The changing size of the dark areas on Mars was often attributed to vegetation and was still regarded as probably in the 1950s. The Mariner probes reversed that opinion with the early images of a cratered Mars looking much like the moon. The Viking missions provided ambiguous indications of surface life in the 1970s. 20 years later, the same happened with the Allen Hills Martian meteorite. Once it was established that early Mars had water, there was a resurgence in the idea that Mars may once have had life. The samples collected for eventual collection and return to Earth may help clarify this. If Mars had life, it is possible that microbial life is extant in the crust where water still exists. Even subsurface caves are a possibility for complex life.
That the Viking mission was to look for life was a gamble, but at least tried. Nasa’s many missions since did everything but look for life, just evidence of previously habitable conditions (“follow the water”). If we start putting boots on Mars in a decade or so, I would hope that part of the effort is doing some deep drilling to get samples that might contain life.
If we do not find life anywhere (extinct or extant) on or in the Martian crust, then this might be indicative of a paucity of life in our galaxy. Exoplanet biosignatures may support of refute that view.
What I do think is that Mars could easily have been contaminated by terrestrial microbes when it was wet, so separate abiogenesis need not have occurred. [Similarly, Venus might have done the same for Earth in its early, pre-greenhouse state.]
I really think we need more ecology education in astrobiology.
Life is local, not planetary. There are extensive areas of Earth that we still struggle to find any life on, deserts and such. Some places in every planet are just not aproppriate to life, and I think near-the-surface regions of the Enceladus oceans are such places.
Fundamentally, there is NO energy flux for biological life to use here. The solar flux hitting the surface cannot be used, it doesn’t exist as far as algae below 10km of ice care. The only reasonable source of usable chemical energy in the subocean are analogues to the hydrothermal vents, where reactive species dissolved from rock become accessible. Life is more likely to be found in the deep areas of the moon than the surface.
Also, energy is a limiting factor in this situation. By a recent PNAS publication, on average the biosphere of Earth uses 5W/kg C. The energy flux would limit the size of the ecology significantly, so it could only survive in small pockets around HT vents.
Phosphorous is very likely not the limiting factor here, so any rock weathering would either accumulate P in large concentrations or crystallise into new rocks, keeping a cycle not that dependent on the biosphere. Maybe it would be possible to evaluate the turnover of P in the ocean with more data points, and use that number to guestimate an energy flux in the possible HT vents or other physical parameters inside the planet.
I beg to differ. Whatever the site of abiogenesis, life has radiated and adapted to a wide range of conditions. Life is more abundant and diverse in teh tropics than the poles. Life sparsely exists in the deserts, wherever water can be gleaned. Sparse life exists in the ocean abyss where water is abundant, but energy is very limited. Life may be absent in newly formed glacial lakes and polluted streams and rivers where both water and energy are abundant. Ecosystems are pockets of interacting communities that have co-evolved. The evolution of new forms requires conditions to change, and options for existing species to radiate new forms when competitive pressures are released.
As that famous phrase expresses – “Life finds a way.”
You seem to forget that we discovered life in the crust forming a huge biosphere,. It may be limited to microbes that can live in the interstices of rock grains, but its estimated biomass is very large, simply because the volume makes up for its sparseness. Microbial life in the cold, abyssal ocean mud has very little food and very slow metabolisms, indicating possibly a millennium to reproduce.
Your argument seems to be very much my point 1 to account for the P if life does exist. Whether life exists in the Enceladan ocean is very speculative. I doubt it but would love to be surprised by a discovery.