How to approach finding life on other worlds will continue to be a challenging issue, but how useful that even as we work out strategies for studying exoplanet atmospheres, we have planets we can actually reach right here in our own Solar System. And if the hunt for life has turned up empty thus far on Mars, we can keep searching there even as we consider the exotic possibility of life in the clouds of Venus. We’ve looked at Venus Life Finder before in these pages. This series of missions is now known as Morning Star, all designed to probe the clouds for signs of a kind of life that would have to endure the most hellish conditions we can imagine. In today’s post, Alex Tolley examines the Morning Star Missions and how they might proceed, depending on the results of that all important first sampling of the atmosphere.
by Alex Tolley
“To boldly seek life, where no terrestrial life has gone before”
The “Morning Star Missions” (formerly Venus Life Finder) group had previously outlined their plans for early life-detecting missions in the possibly habitable, temperate, but highly acidic Venusian clouds, at altitudes of 48-60 km above the searingly hot surface. The first mission, now slated for a 2025 launch, includes an Autofluorescing Nephelometer (AFN) that can detect organic materials, a prerequisite for living organisms. [1] The instrument emits laser light that causes certain carbon bonds to fluoresce and be detected (in this case, 440 nm is the selected detection wavelength). If no organic material is detected in the cloud droplets, that would eliminate life as we know it. However, there would still be ambiguities regarding whether organic material was detected or not as not all organic matter will fluoresce when stimulated by light. Typically aromatic carbon ring structures fluoresce, whilst linear carbon chains do not. A double-membraned cell wall that could contain a prebiotic metabolic system would probably fail to register. This might well be considered a false negative for what could be a very interesting finding.
It is well known that sulfuric acid (H2SO4) has a deleterious effect on organic matter, and highly concentrated sulfuric acid (CSA) that is expected in the Venusian clouds will rapidly break down organic matter and therefore it would appear that terrestrial life would rapidly succumb to this level of acid condition. [An acid bath is a traditional means by which murderers dispose of the victim’s body.] This would seem to rule out life of a terrestrial nature even in the Venusian clouds.
What about a positive result? Carbon aromatic rings that readily fluoresce may be very common in the clouds as simple carbon molecules are converted as the compounds fall towards the hotter surface. Polyaromatic hydrocarbons [PAH] are common in space and it has been hypothesized that they may be common in Venus’ clouds [2].
Apart from the simple destruction of living organisms like plants by pouring CSA onto them, prior work [3] has shown that organic material identified in terrestrial metabolisms is a little more stable than all naturally occurring organic compounds in CSA but far less stable than the space of manufactured organic compounds, as shown in figure 1.
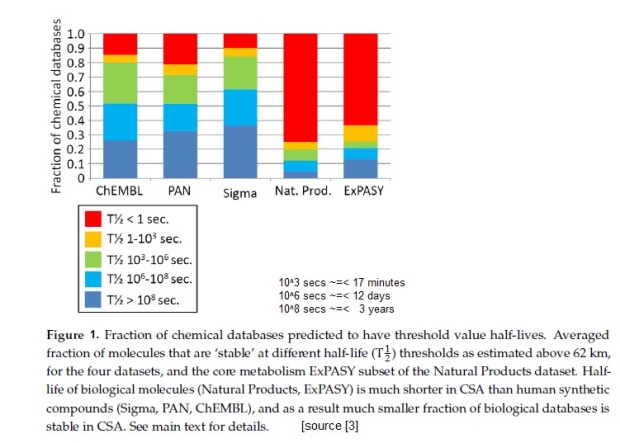
A database of organic compounds and their reactivity to H2SO4 shows compounds with ring structures, especially those with unsaturated carbon-carbon bonds [5]. This implies that any extant organic compounds with these structural features will be more prevalent in the clouds, which includes PAHs. If abiotic aromatic ring carbon compounds are most likely to be resistant to CSA reactions, these abiotic organic molecules may create a false positive result. The search for life must therefore be sure that some biological molecules are resistant to CSA and could theoretically be part of a positive organic molecule detection. Otherwise, this search approach would be futile. That about 10% of the extracted core metabolism compounds are resistant to CSA for greater than 3 years provides support for the possibility that biology may exist in the Venusian clouds.
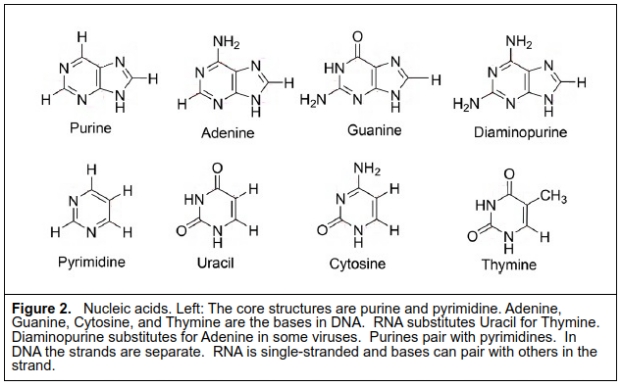
Which biotic molecules are resistant to CSA and therefore could be present in the clouds? An answer to this issue is provided in a new paper by Seager et al in Proceedings of the National Academy of Sciences [4] which examines whether any core biological molecules can survive the acid conditions. Information polymers such as DNA and RNA are a central component of terrestrial life. They are composed of nucleic acids of two types: purines (Adenine, Guanine) and pyrimidines (Cytosine, Thymine, Uracil), linked by a sugar (ribose in RNA, and deoxyribose in DNA) and phosphate. As shown in figure 2, the core structures have unsaturated bonds and very limited exposed bonds that could be attacked by CSA. The purpose of the paper was to determine if these nucleic acids are resistant to CSA and therefore possible detectable molecules on Venus.
The researchers performed several tests, including changes in UV spectra, Nuclear Magnetic Resonance (NMR) to detect changes in C-H bonds, NMR to detect the replacement of the hydrogen atoms with deuterium, and NMR to detect the donation of hydrogen ions, H+, to the nucleic acid molecules by the CSA (protonation).
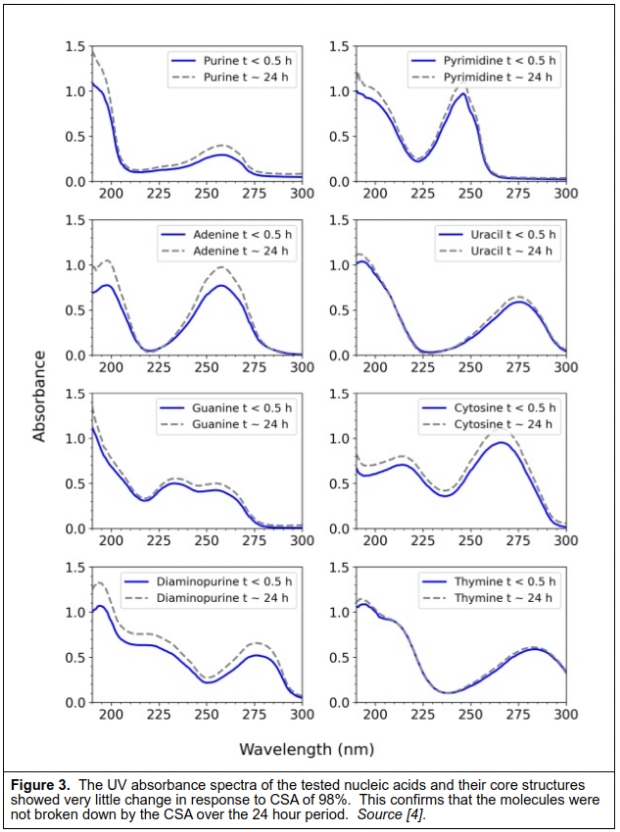
The first series of tests placed these nucleic acids in CSA and tested how the UV spectrum changed over a period of up to 2 weeks in acid concentrations up to 98%. The spectra for the treated nucleic acids were very similar to those in aqueous solutions, indicating that there was no fundamental change in structures or breakdown of the compounds.
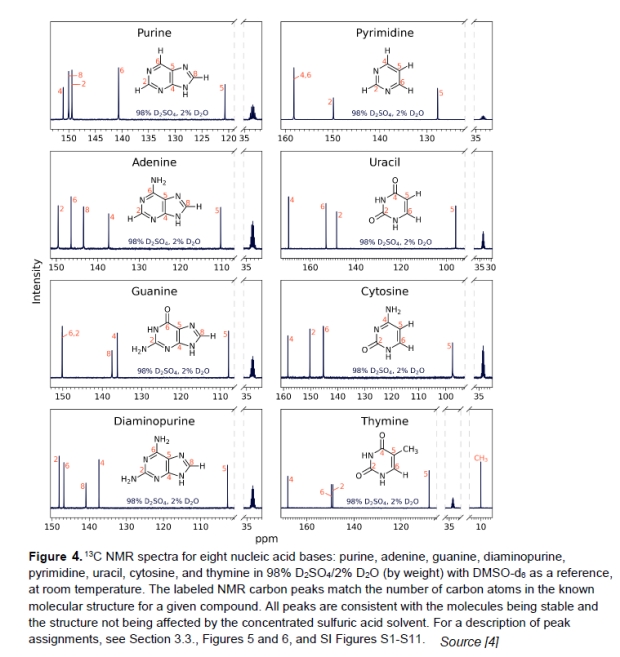
The next series of experiments ran NMR tests on the nucleic acids. This detects the state of the carbon atoms and their bonds which are shown by chemical shifts (ppm) in the hydrogens. Once again, the sharp spectral peaks were very similar to the controls, indicating that the structures and identified carbon and nitrogen bonds had not been changed. Figure 4 shows the results.
The last series of experiments used deuterated sulfuric acid [D2SO4] as well as C13 and N15 isotopes in the nucleic acids to determine if any of the bound hydrogen atoms had been replaced, indicating that the structures were capable of having the C-H bonds broken. Again, there was no evidence of bond-breaking and H atoms replacement.
“Taken together the NMR data confirms that the purine ring structure remains intact in 98% w/w D2SO4 in D2O.“
As the nucleic acids were in CSA where H+ ions were abundant, there is the question of whether these ions protonate the compounds. This protonation of the nitrogen and oxygen atoms was detected by NMR. As hydrogen bonds are important in biological functions, most notably the base pairing between purines and pyrimidines in DNA, and pairing of bases in the same RNA strands, protonation would impact these interactions. Figure 5 shows how protonation disrupts this pairing.
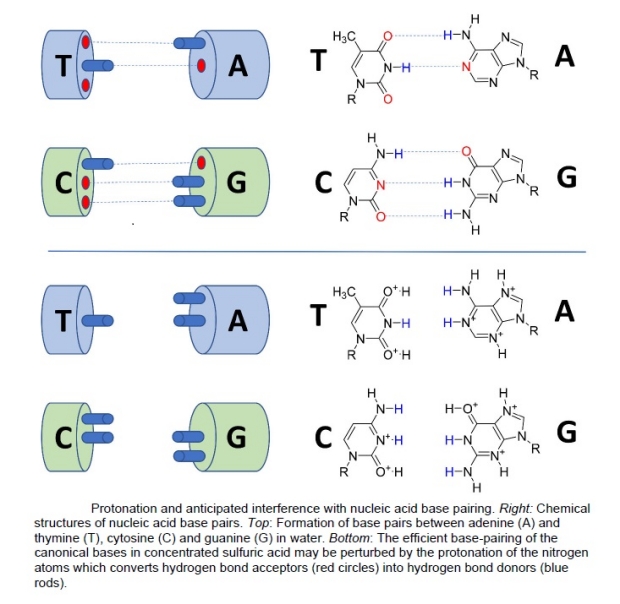
Figure 5. The base pairings in aqueous solutions and the impact of H2SO4 protonation that breaks the hydrogen bonds and pairing. Source: Seager et al 2023 [4]
The conclusion is that the purines and pyrimidines of terrestrial information molecules will remain stable in the Venusian clouds in the habitable region. As these molecules will fluoresce, a positive result of organic molecule detection could include these molecules, but follow-on missions would be needed to determine whether these molecules are present.
In summary, these experiments demonstrate that terrestrial information molecules using the core purine and pyrimidine structures are stable in CSA and therefore could potentially be present in the Venusian clouds. Therefore if organic carbon is detected in the first mission, a 2nd mission to characterize the carbon compounds is supported as the presence of organic carbon could include biological molecules.
While the detection of these nucleic acids would be very interesting, it is important to note that to be useful information molecules, they must polymerize in a way that allows their informational function to operate. Otherwise, the nucleic acids are like an alphabet that cannot be composed in text, as the sugar-phosphate links between them would not be stable in CSA. Other molecules would need to be used. Currently, possible linker molecules have not been identified and remain an area of work.
We already know that amino acids are not stable in sulfuric acid, which rules out proteins as the main functional type of molecule of terrestrial life, existing in the Venusian clouds without some mechanism to neutralize the pH.
If amino acids were stable, could the first mission detect them? Amino acids with cyclic rings such as tryptophan fluoresce, albeit with a peak well below the 440 nm detection wavelength of the AFN to be included in the first mission. If subsequently confirmed by other instruments on later missions, it would indicate that the cloud droplet environment is not as unfavorable as assumed. As a side note, the somewhat controversial detection of phosphine suggests the known rapid oxidation by CSA is at least partially avoided, perhaps by either avoiding the cloud droplets or the droplets having a higher pH, or both.
What are the implications for life if nucleic acids are confirmed and in polymer form? The authors offer 3 scenarios:
1. Life may have emerged during the early wet age in Venus’ oceans. As the planet became the hot dry world it is today, that life could have evolved to adapt to the new cloud-borne, temperate, but concentrated sulfuric acid conditions. The DNA/RNA would have had to change links between the nucleic acids to retain their function.
2. During its evolution to the current conditions, life may have evolved the ability to neutralize the acid by excreting ammonia. This would allow it to retain the existing nucleic acid sugar-phosphate links in DNA and RNA, as well as allow proteins to remain stable.
3. Lastly, the abiogenesis of new life in the clouds. Perhaps this is limited to a pre-biotic state with nucleobases only.
In my opinion, scenario 2 seems most likely if there is evidence that terrestrial-analog cellular life exists in the cloud droplets, using polymerized nucleic acids as their information molecules. This is because we know from the evolution of terrestrial life that core metabolism, information storage, and transcription and translation to functional proteins, have remained almost unchanged over billions of years. Extremophiles have been unable to change their core replication and growth biology, despite adapting to their current environments. What they do instead is tinker with the relative production of certain proteins, and evolve new enzymes and pathways to produce new molecules to adapt to the new conditions. Therefore being able to produce ammonia to neutralize the CSA seems a more likely evolutionary path.
If however nucleic acids are found and in a polymerized, functional state, but without accompanying amino acids and functional proteins, is it possible that Venus is in the equivalent condition of the hypothetical pre-biotic RNA World? In this scenario, RNA acts as both the information and functional molecule. We see evidence for its metabolic function as RNA can act as a catalyst and also autocatalyze itself to replicate. On Venus, the RNA analog may be pre-biotic or possibly degenerate, the remaining functional mechanism in a hostile pH environment. Despite this last speculation, it raises the question “How would these ‘nucleic acid bases’ be formed in the clouds?” While these nucleic acids have been shown to have the ability to form from simple molecules like HCN and formamide in aqueous conditions, there is as yet no evidence that they can form in CSA. Unless they can, this would seem to rule out this pre/post-biotic scenario. (See also Bain paper on H2SO4 as a solvent [3])
In summary, the nucleic acids used in the information molecules DNA and RNA are stable in the acid conditions expected in the Venusian clouds. However, they would not be functional as information molecules unless they can effectively polymerize in a way that allows an analog of the stable form that would allow natural selection to operate. They would need different linker molecules than the sugar-phosphate ones on Earth. Furthermore, protonation of the nitrogens in the nucleic acids would disrupt the hydrogen bonding mechanism for the base pairings. This is another important issue that further constrains the possibility of life on Venus unless it can neutralize the pH of the cloud droplets, with a metabolism that relies on methanogenesis of CO2 like terrestrial archaea, or organic molecules produced in the atmosphere.
If organic molecules are detected in the first 2025 scheduled mission, the stability of nucleic acids in CSA indicates that there is potential for their direct detection in a follow-up mission, holding out the possibility of some sort of life or pre-biotic chemistry on Venus.
References
Tolley, A (2022) “Venus Life Finder: Scooping Big Science” Centauri-Dreams https://centauri-dreams.org/2022/06/03/venus-life-finder-scooping-big-science/
Špaček, J (2021) “Organic Carbon Cycle in the Atmosphere of Venus”, arXiv preprint arXiv:2108.02286.
Bains W, Petkowski JJ, Zhan Z, Seager S. Evaluating Alternatives to Water as Solvents for Life: The Example of Sulfuric Acid. Life (Basel). 2021 Apr 27;11(5):400. doi: 10.3390/life11050400. PMID: 33925658; PMCID: PMC8145300.
Seager, S et al (2023) “Stability of nucleic acid bases in concentrated sulfuric acid: Implications for the habitability of Venus’ clouds” PNAS 2023 Vol. 120 No. 25 e2220007120 https://doi.org/10.1073/pnas.2220007120
Database of H2SO4 effects on molecules. Reactivity V4.1- release.xlsx Url: https://zenodo.org/record/4467868/files/Reactivity%20V4.1-%20release.xlsx



Interesting Alex
While on the subject of Venus this was out today too.
SwRI-LED TEAM FINDS ANCIENT, HIGH-ENERGY IMPACTS COULD HAVE FUELED VENUS VOLCANISM
https://www.swri.org/press-release/swri-led-team-finds-ancient-high-energy-impacts-could-have-fueled-venus-volcanism
Cheers Edwin
I cannot read the paper, but it is interesting as it is very similar to Fred Hoyle’s mid-1950s theory about Earth’s vulcanism. “The Frontiers of Astronomy” (©1955). With little data and no paradigm shift to plate tectonics, Hoyle thought that the clumping of planetesimals might just get to the core temperate of 5000C. Radioactive heating was just limited to Uranium, with no mention of Al-26. Thomas Gold’s theory of pores in the mantle accounted for the fractionation of the iron to the core, as well as volcanoes, ore deposits, and oil and gas deposits. (Hoyle disdainfully dismisses geologists’ claims of biologic oil as “fish decay” is far too little. – clearly supportive of Gold in the text). Hoyle writes in a very engaging style, but with hindsight, one knows that his assumptions and models were incorrect, or at least superseded by better ones.
Without reading the paper I cannot know their model and what their assumptions are. I would want planetologists to discuss this paper as they would know whether the model was realistic or not and whether the assumptions were justified.
What is relevant is what contribution to organic molecules the high level of volcanism on Venus makes, especially coupled with the high surface temperatures. Would they generate sufficient aromatic ring compounds that any detection should be assumed to be non-biological rather than biological? [Especially note the Špaček reference on a carbon cycle in the Venusian atmosphere.] H2SO4 rapidly leaches serpentinite to release Mg which can then sequester CO2. Does this mean that volcanoes emitting gases in the H2SO4 atmosphere primarily may be releasing reduced carbon as CH4, which becomes the source for carbon compounds in the clouds? My knowledge is woefully lacking here, so this is just a [wild-assed] guess. We may not know until the various Venus probes reach the planet in the early 2030s what the effect of the vulcanism has beyond the surface features.
Is this the paper?
Long-lived volcanic resurfacing of Venus driven by early collisions
@Robin – thank you. This certainly seems to be the paper.
Looking through the paper and the supplementary data, it is not really clear how they arrived at the different impact velocities between Earth and Venus. The chart Figure 1 in the Suppl. Data just shows probability distributions of bolide masses based on a simulation. These distributions are used to provide an average velocity of impact. IDK is that is supposed to be descriptive or not, but the 2 values are then used as examples in the paper to show the differences between Earth and Venus. The effect of the collision that resulted in Earth’s Moon, as well as the lack of small moons around Venus seems to be handwaved away in this analysis. I have no idea whether this is reasonable or not, other than the reviewers clearly passed the paper for publication.
Earth life has something to contribute to this topic. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8196465/ describes the adaptations of Acidithiobacillus (known for growth in mine drainage and ‘snottites’). While it is occasionally said to go lower, it tends to be reported to survive at pH 0.5 or 1 once it gets near a lab. It works by a number of mechanisms, including Na+/H+ antiporters to pump acid out of the cytoplasm of the cell. (Most bacteria have more alkaline cytoplasm than their surroundings anyway, to store respiratory energy, same as our mitochontrial matrix, but Acidithiobacillus goes a bit further)
Venus has a bigger problem in that H2SO4’s Pka1 is -2.8. The pKa of H3O+ on the other hand is 0 … usually… but we also see it given as -1.7 in many textbooks. Secret being in the units, which are written to include the water concentration in that second form (M^1 rather than M^2). Which we likely need to consider as water isn’t even the “solvent” on Venus! By my best guess, Venus life needs to beat out the Earthly organisms that (via medieval miner mishaps) inspired the green slimes and black puddings of the role-playing world, by perhaps 1.1 + 0.5 = 1.6 pH units, or 40-fold increase in H+ concentration.
To Venus’ favor, there is the notion that early Venus was comfortably habitable, Earthlike, capable of evolving life that might even have spread out to Earth. (Which, at least, would settle why we can’t find any prokaryotes with incomplete or significantly different genetic code tables) Venus might have lost its life to a bombardment and/or the catastrophic volcanic process that resurfaced all (or most??) of the planet. One wonders whether intelligent life could have had a hand in such a disaster, or if interesting artifacts or fossils in the claimed ancient tesserae ( https://www.nature.com/articles/s41467-020-19336-1 ) might be imaged in some future expecition. If Venus ever had an advanced ecosystem, and if life survived high in the clouds after the initial catastrophe, it would have had a very long time to focus specifically on surviving conditions that only very slowly become more acidic as the hydrogen was lost from the superheated atmosphere.
The Venusian clouds have a pH of around -1.2, far lower than terrestrial acidophiles can tolerate (minimum pH 0?). Active export of protons seems to be their main method of handling low pH as well as buffering in the cytoplasm. Life in acid: pH homeostasis in acidophiles
It would be interesting if such acidophiles could be coaxed to evolve a tolerance in an even lower pH solution or determine whether they have already reached their maximum tolerance. The Morningstar Mission document has suggested that Venusian cloud organisms might produce ammonia to counteract the acid and raise the pH [in the cytoplasm?].
The early missions to detect organic compounds that could be biosignatures seems increasingly compromised by the detection of many basic organic compounds in meteorites and asteroid samples which presumably are abiotic in nature. This means that chirality detection will be needed as abiotic compounds such as amino acids found in meteorites are racemic (approximately equal amounts of both L- and R-rotatory forms). Ideally, more complex molecules should be detected e.g. proteins and DNA/RNA analogs, which so far have not been detected in space.
While I remain skeptical of success, like SETI, we have to look or we are guaranteed to find nothing.
Interesting link! The paper I found didn’t address membrane potential, but this one makes it clear that these bacteria are “depolarized” (unlike most life, they have a positive charge inside the membrane, which forces H+ out of the cell). Both the Na/H exchangers my link mentioned and the K/H exchangers this one describes have the property of bringing in positive charge to replace the protons. Come to think of it, the ammonia you describe (or any basic amino acid) ought to do very well here, because it can take up the H+ into a non-acidic form (NH4+) where the positive charge is still inside the cell to push H+ out.
Key building blocks of life have now been found in meteorites. More smoking guns…
https://www.sciencenews.org/article/all-of-the-bases-in-dna-and-rna-have-now-been-found-in-meteorites
This is an important finding and relevant for both the origin of life on Earth, but also for teh detection of life on Venus. The nature article link is below.
Firstly the detection of the 5 nucleobases. Adenine, Thymine, Cytosine, Guanine, and Uracil were in parts per billion (ppb) amounts. The new technique the authors used was able to detect these small amounts and extended the previous detection of purines and pyrimidines to complete the terrestrial set.
The authors were able to discount contamination from Earth in teh 3 meteorites detected. However, what we would want is detection in pristine samples from space, such as from Ryugu and Bennu.
That these meteorite nucleobases are not from terrestrial sources and of abiotic origin is demonstrated by the detection of many other isomers (same molecular composition, but structurally different) as a result of chemistry, rather than biology.
The authors also state:
So theoretically we have an abiotic source of the nucleobases that could conceivably create ambiguous biosignatures in the Venusian clouds. Fluorescence, and later chromatography, could detect, and identify these molecules, given sufficient amounts for detection. If many isomers were also identified, that would likely suggest that these were not biosignatures.
This leads me back to Lee Crinin’s work we have discussed on CD in the past. Biology restricts the molecules it produces, so that some molecules have very high abundance and others that should appear by chemistry, do not. Thus a sample of living material will have a high abundance of the 5 bases, but no isomers or analogs except small amounts of those that are metabolic intermediates. Chirality will also be very strong, unlike abiotic sources.
Suppose we were able to look ahead to sample return mission from Venus. Even if any extant life in the sample had died and decayed, an analysis would show that either the molecules detected showed the hallmarks or life, or that they were clearly the result of abiotic chemistry as we see in these meteorite analyses.
A final thought. Suppose we get a sample from deep inside an interstellar object that may have been ejected from another system. Would it have the same sort of distribution of molecules as found in our system, or would there be differences? Would it be possible that they showed distinct biosignatures as a result of origin or contamination from a living world?
Identifying the wide diversity of extraterrestrial purine and pyrimidine nucleobases in carbonaceous meteorites
The Planetary Society takes a shot at answering questions about life on Venus…
https://www.planetary.org/articles/life-on-venus-your-questions-answered
I read an article by Sara Seager et al https://www.liebertpub.com/doi/10.1089/ast.2020.2244
and they describe the acidity of Venusian clouds as follows, to wit: on Earth, extremophiles live in water diluted with sulfuric acid, on Venus, the droplets are sulfuric acid diluted with a bit of water…
“We cannot emphasize enough that the venusian sulfuric acid clouds are much more acidic than even the most harshly acidic conditions found on Earth—the Dallol Geothermal Area, within the Danakil Depression in Northern Afar (Ethiopia) (Cavalazzi et al., 2019)…
Acidity functions are on a log scale, so the clouds of Venus are >10^11 times as acidic as the Dallol geothermal area. This supports our statements that the venusian cloud drops are an entirely different environment from any found naturally on Earth.”
Seager’s logic is impeccable, but the assumptions on which it rests should be better tested. Bains, 2023 ( https://arxiv.org/ftp/arxiv/papers/2306/2306.07358.pdf ) argues that the commonly described pure sulfuric acid droplets are inferred, not observed; he has previously published about anomalies up to and including the presence of ammonia. https://arxiv.org/ftp/arxiv/papers/2112/2112.10850.pdf If the droplets are partially neutralized and contain a substantial fraction of HSO4-, then the dramatic difference described by Seager mostly goes away. The pKa1 of H2SO4 is very low, so it is still very unlikely to match Earthlike conditions, but life might have a better shot. Until we have probes collect these droplets directly, it is tempting to imagine all sorts of possibilities. Is the water so rare in the atmosphere because droplet organisms are hoarding whatever is left inside their cells? :)
The ammonia paper that was also published in PNAS [https://www.pnas.org/doi/10.1073/pnas.2110889118] at the same time is an attempt to make plausible a hypothesis that Venusian cloud organisms could produce NH3 to neutralize the H2SO4 and increase the pH to levels tolerated by terrestrial extremophiles.
It is interesting that the only terrestrial examples of such NH3-producing organisms in the paper are not acidophiles. Obviously, it would be pointless for free-living prokaryotes living in an acidic H2SO4 environment to try to neutralize the environment. However, if acidophiles were living in constrained spaces like the interstices of rocks or in layered biofilms, this might be an option. On Earth, are the acidophiles adapted to the lowest pH natural environments, or have they failed to evolve in more extreme lower pH ones? [My earlier comment about trying to evolve such organisms in increasingly concentrated H2SO4 could be instructive.]
The 2023 paper published in Astrobiology is a wider perspective on possible avenues for organisms to survive in the clouds. It is a justification for searching for life, most notably for the RocketLab-delivered instrument package to Venus that has been described in prior CD posts.
Whether any of the proposed mechanisms could allow for life remains to be seen, it seems worth making the attempts to look as the Venusian clouds are reachable and even allow for sample returns without requiring subsurface drilling such as might be needed on Mars, and certainly on Europa. If life is extant in the clouds, its biology would be extremely valuable to study, and could well have commercial value, although we would need to be careful to prevent accidental escape in case it resulted in some “Andromeda Strain” scenario.
The DAVINCI+ mission scheduled for a 2031 Venus arrival is dedicated to analyzing the atmosphere and presumably will also be able to do some analysis of the cloud droplets. If the first MorningStar Mission fails to detect fluorescing organic molecules in the clouds, DAVINCI might be the next mission to Venus. If the MorningStar missions proceed with the proposed habitability balloon missions, then the DAVINCI mission would provide complementary data on the atmosphere and clouds. In a decade we might know a lot more about the Venusian atmosphere than we do today, hopefully resolving the unexplained anomalous data we currently have.
So far as I know the most acidic environments are either from mine drainage or from caves passing through sulfide-containing minerals or otherwise receiving hydrogen sulfide, such as Cueva de Villa Luz. In these environments, the overall impact of life forms is to release acidity and create an extreme environment. This at least mostly involves creating sulfuric acid (in concept, though they are interacting primarily with sulfate ions) There are a wide variety of life forms and apparently the acid-producing ones are not the most common. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4438317/ The organisms do have limits to what they can tolerate, which gives the impression of a chemical arms race. I have no idea if that race has gone as far as it conceivably could.
Venusian, venereal, aphrodisiac and cytherean: which is correct? – Google Search
Hence Botticelli’s painting: The Birth of Venus
Also Vonnegut’s fictional author, Kilgore Trout’s fictional book Venus on the Half Shell which was actually subsequently written by Philip José Farmer. Very meta.
I actually like Cytherian instead of Venusian, but it is so different a name that it could be confusing. Clarke uses Hermian to describe the Mercurians in Rendezvous with Rama, so the use of Greek naming is certainly within bounds. Now if only Pluto had been named after that Greek God’s earlier name, Hades. However, as Hadean has been taken for the early Earth eon, perhaps that is as well given that Pluto is very cold, not hellishly hot. Had the ancients known Venus’ true nature, Hades and Hadean would have been better naming for Venus. OTOH, Dante might have approved of Hades for Pluto, as his “Inferno” depicts the Devil encased in ice at the center of the underworld. Hell has indeed frozen over, so unlike the more modern Christian depiction of Hell as more Venusian in character.