The exoplanet K2-18b has been all over the news lately, with provocative headlines suggesting a life detection because of the possible presence of dimethyl sulfide (DMS), a molecule produced by life on our own planet. Is this a ‘Hycean’ world, covered with oceans under a hydrogen-rich atmosphere? Almost nine times as massive as Earth, K2-18b is certainly noteworthy, but just how likely are these speculations? Centauri Dreams regular Dave Moore has some thoughts on the matter, and as he has done before in deeply researched articles here, he now zeroes in on the evidence and the limitations of the analysis. This is one exoplanet that turns out to be provocative in a number of ways, some of which will move the search for life forward.
by Dave Moore
124 light years away in the constellation of Leo lies an undistinguished M3V red dwarf, K2-18. Two planets are known to orbit this star: K2-18c, a 5.6 Earth mass planet orbiting 6 million miles out, and K2-18b, an 8.6 Earth mass planet orbiting 16 million miles out. The latter planet transits its primary, so from its mass and size (2.6 x Earth’s), we have its density (2.7 g/cm2), which class the planet as a sub-Neptune. The planet’s relatively large radius and its primary’s low luminosity make it a good target to get its atmospheric spectra, but what also makes this planet of special interest to astronomers is that its estimated irradiance of 1368 watts/m2 is almost the same as Earth’s (1380 watts/m2).
Determining an exosolar planet’s atmospheric constituents, even with the help of the James Webb telescope, is no easy matter. For a detectable infrared spectrum, molecules like H2O, CH4, CO2 and CO generally need to have a concentration above 100 ppm. The presence of O3 can function as a stand-in for O2, but molecules such as H2, N2, with no permanent dipole moment, are much harder to detect.
The Hubble telescope got a spectrum of K2-18b in 2019. Water vapor and H2 were detected, and it was assumed to have a deep H2/He/steam atmosphere above a high pressure ice layer over an iron/rocky core, much like Neptune. On September 11 of this year, the results of spectral studies by the James Webb telescope were announced: CH4 and CO2 were found as well as possible traces of DMS (Dimethyl sulfide). No signal of NH3 was found. Nor was there any sign of water vapor. The feature thought to be water vapor turned out to be a methane line of the same frequency.
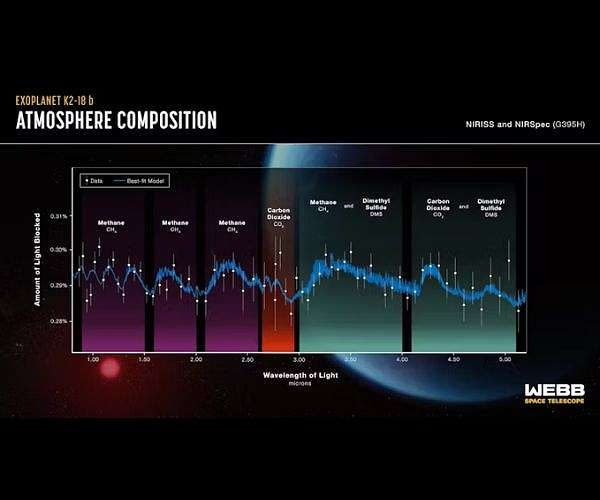
Figure 1: Spectra of K2-18b obtained by the James Webb telescope
This announcement resulted in considerable excitement and speculation by the popular press. K2-18b was called a Hycean planet. It was speculated that it had an ocean, and the possible presence of DMS was taken as an indication of life because oceanic algae produce this chemical. But that was not what intrigued me. What caught my attention was the seemingly anomalous combination of CH4 and CO2in the planet’s atmosphere. How could a planet have CH4, a highly reduced form of carbon, in equilibrium with CO2, the oxidized form of carbon? A search turned up a paper from February 2021: “Coexistence of CH4, CO2, and H20 in exoplanet atmospheres,” by Woitke, Herbort, Helling, Stüeken, Dominik, Barth and Samra.
The authors’ purpose for this paper was to help with the detection of biosignatures. To quote:
The identification of spectral signatures of biological activity needs to proceed via two steps: first, identify combinations of molecules which cannot co-exist in chemical equilibrium (“non-equilibrium markers”). Second, find biological processes that cause such disequilibria, which cannot be explained by other physical non-equilibrium processes like photo-dissociation. […] The aim of this letter is to propose a robust criterion for step one…
The paper presents an exhaustive study for the lowest energy state (Gibbs free energy) composition of exoplanet atmospheres for all possible abundances of Hydrogen, Carbon, Oxygen, and Nitrogen in chemical equilibrium. To do that, they ran thermodynamic simulations of varying mixtures of the above atoms and looked at the resulting molecular ratios. At low temperatures (T ≤ 600K), they found that the only molecular species you get in any abundance are H2, H20, CH4, NH3, N2, CO2, O2. At higher temperature, the equilibrium shifts towards more H2, and CO begins to appear.
Some examples of their results:
If O > 0.5 x H + 2 x C ––> O2-rich atmosphere, no CH4
If H > 2 x O + 4 x C ––> H2-rich atmosphere, no CO2
If C > 0.25 x H + 0.5 x O ––> Graphite condensation, no H20
They also used the equations to tell what partial pressures of the elemental mixture will produce equal pressures of the various molecules:
If H = 2 x O then the CO2 level will equal CH4
If 12 C = 2 x O + 3 x H then the CO2level will equal H20
If 12 C = 6 x O + H then the H20 level will equal CH4
To summarize, I quote from their abstract:
We propose a classification of exoplanet atmospheres based on their H, C, O, and N element abundances below about 600 K. Chemical equilibrium models were run for all combinations of H, C, O, and N abundances, and three types of solutions were found, which are robust against variations of temperature, pressure, and nitrogen abundance.
Type A atmospheres[which] contain H20, CH4, NH3, and either H2 or N2, but only traces of CO2 and O2.
Type B atmospheres [which] contain O2, H20, CO2, and N2, but only traces of CH4, NH3, and H2.
Type C atmospheres [which] contain H20, CO2, CH4, and N2, but only traces of NH3, H2, and O2…
Type A atmospheres are found in the giant planets of our outer solar system. Type B atmospheres occur in our inner solar system. Earth, Venus and Mars fall under this classification, but we don’t see any planets with Type C atmospheres.
Below is a series of charts showing the results for each of the six main molecular species over a range of mixtures.
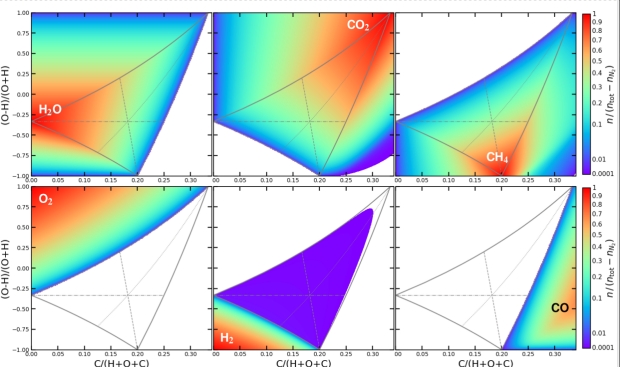
Figure 2: The vertical axis is the ratio of Hydrogen to Oxygen, starting at 100% Hydrogen at the bottom and running to 100% Oxygen at the top. The horizontal axis shows the proportion of Carbon in the total mixture (The ratio runs up to 35%.) Molecular concentrations are in chemical equilibrium as a function of Hydrogen, Carbon, and Oxygen element abundances, calculated for T = 400 K and p = 1 bar. The blank regions are concentrations of < 10−4.
The central grey triangle marks the region in which H20, CH4, and CO2 can coexist in chemical equilibrium. The thin grey lines bisecting the triangle indicate where two of the constituents are at an equal concentration. These lines are hard to discern unless you can magnify the original image. For H20 and CO2 at equal concentration, it’s the dashed line (the near vertical line running upwards from 0.2 on the horizontal scale.) For CO2 and CH4, it’s the horizontal line. And for H20 and CH4, it’s the dotted line swooping upwards toward the top right-hand corner.)
The color bars at the right-hand side of the charts are both a color representation of the concentration and show the proportion of Nitrogen tied up as N2, i.e. that which is not NH3. Not surprisingly, the more Hydrogen there is in the mix, the higher the proportion of NH3 there is.
Other Results from the Paper
In the area around the stoichiometric ratio for water you get maximum H20 production and supersaturation occurs. Clouds form and the water rains out. Therefore, you cannot get an atmosphere with very high concentrations of water vapor unless the temperature is over 650°K, the critical point of water. Precipitation results in the atmospheric composition moving out of the area that gives CO2/CH4 mixtures.
Atmospheres with high carbon concentrations and having Hydrogen and Oxygen near their stoichiometric ratio have most of the atmospheric constituents tied up as water, so at a certain point carbon forms neither CO2 nor CH4 but rains out as soot. This, however, only precludes mixtures in the very right hand side of the CO2/CH4 Triangle.
Full-equilibrium condensation models show that the outgassing from warm rock, such as mid-oceanic ridge basalt can naturally produce Type C atmospheres.
Thoughts and Speculations
i) While it is difficult to argue with the man who coined the term, I still think Madhusudhan’s description of K2-18b as Hycean is too broad. Watching Madhusudhan in a Youtube interview, he refers to his paper “Habitability and Biosignatures of Hycean Worlds,’ which suggests that ocean covered planets under a Hydrogen atmosphere can exists within a zone that reaches into a level of irradiance slightly greater than Earth’s; however, he doesn’t mention the work by Lous et al in their paper, “Potential long-term habitable conditions on planets with primordial H–He atmospheres,” that showed that inside irradiance levels equivalent to 2 au from our Sun or greater, the Hydrogen atmosphere required to maintain Earthlike temperatures and not cook it is so thin that it is lost quickly over geological timescales. (You can see this in more detail in my article Super Earths/Hycean Worlds.) I would therefore define a Hycean planet as a rocky world with a radius up to 1.8 x Earth’s outside the irradiance equivalent of 2 au from our sun. K2-18b, being both larger than this and less dense than a rocky world, would fall, in my mind, firmly into the category of sub-Neptune.
ii) Another way of thinking of Type A, Type B and Type C atmospheres is to denote them as Hydrogen dominated, Oxygen dominated and Carbon dominated. Carbon dominated atmospheres may have by far the bulk of their constituents being Hydrogen and Oxygen; but because the enthalpy of the Hydrogen-Oxygen reaction is so much greater than the other reactions, when Hydrogen and Oxygen are close to their stoichiometric ratio, they preferentially remove themselves from the mix leaving Carbon as the dominant constituent. There is no Nitrogen dominated atmosphere because for most of its range Nitrogen sticks to itself forming N2 and is inert.
iii) The lack of H20 spectral lines is puzzling. Madhusudhan in his interview suggests that the spectra was a shot of the high-dry stratosphere. To cross-check the plausibility of this, I looked up the physical data on DMS. Dimethyl Sulfide vaporizes at 37°C and freezes at -98°C, which is lower than CO2’s freezing point. It also has a much higher vapor pressure than water at below freezing temperatures, so this does not contradict the assumption.
iv) I’m surprised this paper is not more widely known as not only does it provide a powerful tool for the analysis of exosolar planets’ atmospheric spectra, but it can also point to other aspects of a planet.
After the Hubble results came out in 2017, papers were published to model the formation of K2-18b, and while a range of possibilities could match the planet’s characteristics, they all came from the assumption that the planet began via the formation of a rocky/iron core followed by the gas accretion of large amounts of H2, Helium, and H20. According to the coexistence paper though, you cannot have large amounts of H2 and get a CO2/CH4 mix with no NH3. So to arrive at this state, this planet must never have had much gas accretion in the first place, or lost large amounts of Hydrogen after it formed. This latter scenario would require the planet to gain a Hydrogen envelope while at less than full mass in a hot nebula and then at full mass, in a cooler environment, lose most of its Hydrogen.
It is much easier to explain the planet’s characteristics by assuming it formed outside the snowline, never gained much of a gas envelope in the first place and spiraled into its present position. If it was formed from icy bodies like Ganymede and Titan (density ~ 1.9 gm/cc), this would give a good match for its density (2.7 gm/cc) allowing for gravitational contraction. The snow line is also the zone where carbonaceous chondrites form, so this would give the planet a higher carbon content than a pure rocky/iron one.
v) Madhusudhan, again from his interview, seems to think that K2-18b is an ocean planet, but I’m dubious about this for two reasons:
The first is that from the work done on Hycean planets by Lous et al, any depth of atmosphere especially with the potent greenhouse mix of CO2 and CH4 is likely to result in a runaway-greenhouse steam atmosphere inside the classically defined habitable zone (inside 2 au. for our sun).
The planet’s CO2/CH4 mix also points against this. From the paper, if there is a slight excess of Hydrogen over the stoichiometric ratio for water, then condensing H20 out, as either water or high pressure ice, pushes the planet’s atmosphere towards a Type A Hydrogen excess with no CO2 and NH3 lines appearing.
All of this would point towards a planet with a rocky/iron core overlaid by high pressure ice, which would, at about the megabar level, transition to a gas atmosphere composed mainly of super-critical steam. This would make up a significant volume of the planet. At the top of this atmosphere, the water, now in the form of steam, would condense out as virago rain leaving a dry stratosphere consisting mainly of CO2, CH4, H2 and N2.
To test my assumption, I did a rough back of the envelope calculation using online calculators, and looked at the wet adiabatic lapse rate (the rate of increase in temperature when saturated air is compressed) per atm. pressure doubling starting from 1 bar at 20°C. This rate (1.5°C/1000 ft) is considerably less than the rate for dry gases (3°C/1000 ft).
It was all very ad hoc, but the first thing I noted was that for each pressure doubling, the boiling point of water goes up significantly–at 100 bar, water boils at 300°C–until its temperature approaches its critical point (374°C) where it levels off. So the lapse rate increase in temperature chases the boiling point of water as you go deeper and deeper into the atmosphere; however, from my calculations, it catches water’s boiling point at 270°C and 64 bar. The calculations are arbitrary—I was using Earth’s atmospheric composition and gravity–and small changes in the parameters can result in big changes in the crossover point; but what this does point to is that if the planet has an ocean, it could be a rather hot one under a dense atmosphere, and if the atmosphere has any great depth then the ocean is likely to be a supercritical fluid.
Also, for the atmosphere to be thin, the planet’s ratio of CO2, CH4 and H2 must be less than 1/10,000 that of H20, which is not something I regard as likely, given what we know about the outer solar system.
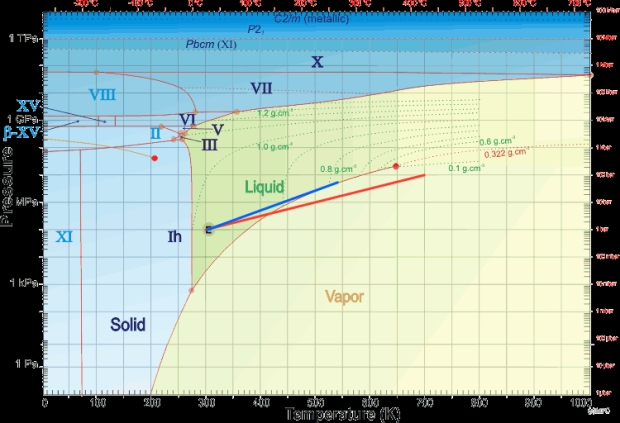
I’ll leave you with a phase diagram of water with (red line) the dry adiabat of Venus moved 25°C cooler to represent a dry Earth and the wet adiabat (blue line) the one I calculated out. It’s also a handy diagram to play with as it gives you an idea of how deep the ocean or critical fluid layer will be at a given temperature before it turns into a layer of high pressure ice.
vi) One final point, and this reinforces the purpose of the paper: that we need to thoroughly understand planetary chemistry to eliminate false bio-markers. DMS is widely touted as a biomarker, but if we look at the most thermodynamically stable forms of sulfur: In a Type A reducing atmosphere, it’s H2S; and in a wet, oxidizing, Type B atmosphere, it’s the Sulfate (SO42-) ion. Unfortunately, the authors of the paper did not extend their thermodynamic analysis to Sulfur, but if we look at DMS’s formula (CH3)2S, it looks an awful lot like a good candidate for the most thermodynamically stable form of Sulfur for a Type C atmosphere, not a biomarker.
References
Wikipedia: K2-18b
https://en.wikipedia.org/wiki/K2-18b
N. Madhusudhan, S. Sarkar, S. Constantinou, M Holmberg, A. Piette, and J. Moses, Carbon-bearing Molecules in a Possible Hycean Atmosphere, Preprint, arXiv: 2309.05566v2, Oct 2023
https://esawebb.org/media/archives/releases/sciencepapers/weic2321/weic2321a.pdf
P. Woitke, O. Herbort, Ch. Helling, E. Stüeken, M. Dominik, P. Barth and D. Samra, Coexistence of CH4, CO2, and H2O in exoplanet atmospheres, Astronomy & Astrophysics, Vol. 646, A43, Feb 2021
https://doi.org/10.1051/0004-6361/202038870
N. Madhusudhan, M. Nixon, L. Welbanks, A. Piette and R. Booth, The Interior and Atmosphere of the Habitable-zone Exoplanet K2-18b, The Astrophysical Journal Letters, 891:L7 (6pp), 2020 March 1
https://doi.org/10.3847/2041-8213/ab7229
Super Earths/Hycean Worlds, Centauri Dreams 11 November, 2022
Youtube interview of Nikku Madhusudhan, Is K2-18b a Hycean Exoworld? on Colin Michael Godier’s Event Horizon



IIUC, what you are saying is that an atmosphere with CH2, CH4 with possible DMS which would be considered as a biosignature, is quite possibly/probably an abiotic condition.
Ambiguity once again.
The CH4 would likely rule out an O2 atmosphere too, so any source of DMS would not be from some phytoplankton like algae, which would then suggest no chlorophyll “red edge” to look for in the optical spectrum.
If this was a terrestrial-type world, would we be happy with the CH4, CO2 presence in the atmosphere as indicative of a biosignature for a planet in our archaean eon, with methanogens generating the bulk of the CH4?
CH2 should be CO2
When thinking about Type A, B, C atmospheres, it is best to think of them as Hydrogen, Oxygen and Carbon dominated atmospheres. In a Hydrogen dominated atmosphere, Hydrogen combines with Oxygen and Carbon. You get H20 and CH4. In an oxygen dominated atmosphere, Oxygen combines with everything else. You get H2O and CO2. In a Carbon dominated atmosphere, Carbon combines with everything else. You get CO2 and CH4 (Note: due to the higher enthalpy of H2O, Hydrogen and Oxygen preferentially take themselves out of the mix first.)
So when considering Sulfur in the equation, in a Type C atmosphere, it will be preferentially combined with Carbon as much as possible. I had considered Carbon disulfide as a possibility. It has fairly similar properties to DMS; however, CS2 has two Sulfurs per Carbon whereas DMS (S(CH3)2 has two Carbons per Sulfur. It therefor looks to me, more and more, like DMS is the equilibrium abiotic compound formed in Carbon dominated atmospheres. Methane on Earth may be considered a sign of life. Methane on Jupiter—not.
There were some other illustrations from this paper that I did not show, but one of them had the trajectory of Earth’s chemical evolution. Earth started towards the bottom left hand corner with a Hydrogen dominated atmosphere and migrated to the top lefthand corner with a Oxygen dominated atmosphere. In the course of this transition, it looks like, that for a period, it passed through a phase of having a Carbon dominated atmosphere, so finding a CO2 and CH4 together in a terrestrial planet atmosphere may not be indicative of biological activity; however, if there’s Oxygen there as well, that would be a sign of chemical disequilibria.
Some folks really want some habitable sub-Neptunes-sized planets. It is admittedly depressing to think that the vast majority of planets more than 3 times Earth’s mass and 1.3 times its radius are basically mini-ice giants or mini-gas giants.
It sounds like the only way you’d get a true Hycean planet would be if it formed in the outer solar system with a mass below that threshold (so it can’t hold on to any meaningful atmospheric hydrogen), before migrating inward. Nobody is particularly excited about that possibility, though, because it’s so obviously inferior as a life-bearing candidate compared to an Earth-like planet of the same size.
Brett, I basically agree; although, I suspect that systems can have varying degrees of migration. Our solar system appears not to have had that much.
Any Hycean planet in the range that you talked about, if formed outside the snowline would have a lot of water, which would mean a deep ocean over high pressure ice.
There are, however, some interesting possibilities for planets that form inside the snowline, particularly for Red Dwarfs. Red Dwarfs fall longer down their Hayashi tracks than sunlike stars before Hydrogen fusion kicks in and puts them on the main sequence. This means that at any given point from the star the initial nebula temperatures is higher relative to its final temperatures than for our solar system. In our solar system, the ratio of the habitable zone to the snowline is Earth to Jupiter 1:5. For a star like K2-18, it’s more like 1:15. K2-18b at 0.16 au to a distance 2.5 au. (This assumes the snowline distance scales 1:1 with stellar mass.)
If a planet, slightly larger than Earth forms inside 2 au, which would make it terrestrial, and does not migrate further in than 0.32 au. (The Hycean inner limit for K2-18) then you could get a Hycean planet with continents and oceans, which would have the potential to be life bearing.
The Spectra of K2-18b show not any water or oxygen. Therefore there is not any water or any oxygen. We can assume that this planet has enough mass or gravity to keep some hydrogen and a thick atmosphere. Volcanism would explain the CH4 and CO2. I agree with Brett that it is something like a gas giant with a lot of atmosphere and big greenhouse but no water. Methanol forms under high atmospheric pressures. Abiotic Dimethyl sulfide can be made by combining iodine with dimethyl disulfide with iodine and methanol. This could be complex chemistry and false positives, but not life. The production of these increase with temperature. Google source.
Maybe it is some primitive, anaerobic life which does not use oxygen?
Geoffrey, This planet has water. We’re just not seeing it.
It’s density indicates that. The only other way to get such a low density is a very deep Hydrogen atmosphere, and if that were the case, you’d have a Type A atmosphere with NH3 lines (which they would have detected) and no CO2.
Also, if the planet accreted that much Hydrogen, why didn’t it accrete some water vapor as well?
Volcanism does produce CO2 and CH4, but the main gas it emits is water vapor.
The three gases detected CO2, CH4 and DMS are all more volatile than water. I think we’re not seeing the water because we’re seeing the cold upper atmosphere with all the water is frozen out.
Why would we be seeing only the upper atmosphere? The JWST used transmission spectroscopy to obtain the above spectra which involves the planet passing in front of the star. With transmission spectroscopy, the starlight goes through all parts of the atmosphere where an absorption spectrum is taken. Uranus and Neptune have that problem with the ice inside, but K2-18b is in the life belt, the habitable zone, so there should be water vapor. If we don’t see any, I doubt the planet is an ocean planet. CH4, carbon one molecule and hydrogen four molecules means that there is hydrogen there. I still think it is weird chemistry since the our understanding of mini Neptune’s is pioneer work, but that also means we need to know more so I will admit the Jury might be still out on the water on the planet. Until we have better evidence, I would not give water a high probability. The planet is also tidally locked, but a big green house effect might effect that so there should be some water vapor on the sunlight side and terminator.
Perhaps the water was uv photo disassociated into hydrogen which filled the atmosphere and the oxygen combined with other elements to form oxide compounds.
Hi David
Another really interesting post and I did enjoy your previous one too, do you have any more diagrams of this Planet?
I was also reading this one tonight too
No Evidence for More Earth-sized Planets in the Habitable Zone of Kepler’s M versus FGK Stars
https://arxiv.org/abs/2310.11613
Thanks Edwin
Edwin, I don’t have any more diagrams of the planet. The paper had other diagrams, one showing that the atmosphere becomes saturated with water around its stoichiometric ratio and the water rains out if the temperature is below water’s critical point. At high carbon concentrations, soot also rains out around water’s stoichiometric ratio. At about 48 kilobar, which is above the high pressure ice line if the temperature is above water’s critical point, the soot turns into diamonds.
The other diagram tracks the condensation histories of various nebula dust types as they cool. I didn’t go into this as it wasn’t particularly applicable to K2-18b and I didn’t want the article too get any longer.
Thank you for that paper recommendation. I’ve downloaded it but not read it yet.
Venus is a good example of solar wind stripping and the lose of water in planets that don’t have a magnetic field. A tidally locked planet most likely does not have a magnetic field because without a fast rotation, there can be no charged particles that move in circles which is needed to generate a magnetic field to block the solar wind and trap particles in the magnetic field. Consequently, Red dwarf worlds around older stars might have little or no water. The atmospheric chemistry of K2-18B, the hydrocarbons methane, and dimethyl sulfide plus carbon dioxide might be what is left over from atmospheric stripping?
This exoplanet is large enough to trap a considerable atmosphere so it might have a warm temperature and big green house effect. There are also other means of water loss such as photolysis or photodissociation of water into hydrogen and oxygen where water is lost to space. I got the idea from the article Water World’s Don’t Stay Wet for Long, Matt Williams, Universe Today.
Geoffrey, we know the planet has some Hydrogen in its atmosphere; its spectral signature was detected, but we also know it can’t have too much, otherwise no CO2 and you’d get NH3 lines. So the question is how does your model account for the planet’s density 2.7 gm/cc.
From what the literature says about K2-18b is that it is thought to be a rocky planet. 2.7 gm/cc.would include silicates. Both Dimethyl sulfide C2H6S and CH4 are hydrocarbons. Assuming the planet is tidally locked and tidal heating and a hot interior these chemicals can be replenished. Maybe the planet does not have any ammonia NH3 and therefore maybe no life. Quote by Dave Moore: “N2, with no permanent dipole moment, are much harder to detect. ” There may be N2, but it may be the life is needed to make NH3 from N2 at least a spectroscopically detectable spectrum of NH3. This is what happened on Earth.
Good synopsis on K21b.
Now I’d like to see a CD article on Hd 139139.
I’m tempted to discuss some of that thermodynamics as a learning exercise. Using Sara Seager’s AllMols.Org database (Thanks to Alex Tolley and Paul Gilster for finding the new link for this site!) we can find oodles of data about all these compounds. The paper uses an equilibrium temperature of K2-18b of 250K to 300K, and lists an H2-rich atmosphere at ~300 K as one possibility. But the depth at which the transit occurred, the temperature of the atmosphere, not to mention the winds that might bring DMS up to be observed… all depend on the model of the atmosphere. I suppose there’s a reason why the authors of the actual paper ran elaborate simulations.
Nonetheless, if we imagine a reaction (doesn’t have to be doable; this is thermodynamics…)
Equation: H2S + 2CH4 = CH3SCH3 + 2H2
SMILES: S C CSC HH
deltaG 300K: -3.00 -50.70×2 +7.77 -0.05×2
deltaG1500K: 156.40 +71.40×2 +272.52 +0.30×2
The change in overall delta G at 300K for this reaction is +112.07 kcal/mol. That’s a huge amount of free energy that would need to be put into this reaction to make it go forward, if all components were at 1 atm concentration. We still need to modify this by RT ln Q – if H2 is very rare in the atmosphere, or H2S and/or CH4 very common, then it could be at equilibrium… I dunno. Something is busting up perfectly good CH4 molecules into CH3 groups and leftover hydrogen. It could be life…
But it could just be some hot internal layers of the atmosphere. The same calculation for the 1500K column yields -25.68 kcal/mol. Unless I fouled up (all too likely) this is actually an exergonic (spontaneous) reaction at 1500 K, mostly because the methane is getting shaken apart in the heat. This might conceivably happen in a deep supercritical atmosphere more like Neptune’s; but the temperature required for dimethylsulfide to prevail at equilibrium should be less deep in a type C atmosphere, high in CH4 and lacking in H2.
Try adding CO2 to equation and H2
CO2 + H2S + CH4 +2H2 –>CH3SCH3 + 2H2O
What do you get?
Interesting chemistry, but solar wind stripping, photo dissociation seem to have caused any original water to have been lost and the rate of those chemical reactions might not be fast enough to replenish the water otherwise we would see it in the spectra. There may be some water there at the molecular level, but it looks bone dry like Venus atmosphere.
Equation: CO2 + H2S + CH4 +2H2 –>CH3SCH3 + 2H2O
SMILES: O=C=O, S, C, [H][H], CSC, O
deltaG298K: -395, -3, -51, 0, 7, -245* (-449 -> -483)
deltaG1500K: -398, 156, 71, 0, 272, -248 (-171 -> -224)
Interesting idea! It looks like both reactions should occur spontaneously, at least under standard conditions. The first delta G isn’t really accurate because the water then condenses; we normally wouldn’t include a liquid water term in the equilibrium constant, but the vapor pressure must be far below 1 atm driving the reaction forward anyway. I was suspicious other reactions would destabilize your atmospheric mixture, but at least trying to make SO2 and CH2O didn’t immediately give me exergonic reactions. I could learn a lot of chemistry playing with this data!