The first generation of stars in the universe began to shine in an era when chemical elements like carbon and oxygen were not available. It was the explosion of these early stars in supernovae that began the process of enrichment, with heavier elements fused in their cores now spreading into the cosmos. Lower-mass stars and planetary systems began to appear as heavier elements could form the needed dust grains to build planetary cores.
Avi Loeb (Harvard-Smithsonian Center for Astrophysics) and grad student Natalie Mashian have been looking at a particular class of ancient stars called carbon-enhanced metal-poor (CEMP) stars. Here the level of iron is about one hundred-thousandth as high as our Sun, a clear marker that these stars formed before heavy elements were widely distributed. These stars are interesting because despite their lack of iron and other heavy elements in comparison to the Sun, they are rich in carbon, an excess that leads to the possibility of planets forming around them out of clumping carbon dust grains.
The new paper on this work looks at the possibility of carbon planet formation, pointing to early work that has simulated such planets, and observational indications of planets with carbon-rich atmospheres (WASP-12b) and carbon-rich interiors (55 Cancri e). If they’re out there, finding such planets — made of graphite, carbides and diamond — around CEMP stars could be a productive exercise. “These stars are fossils from the young universe,” explains Loeb. “By studying them, we can look at how planets, and possibly life in the universe, got started.”
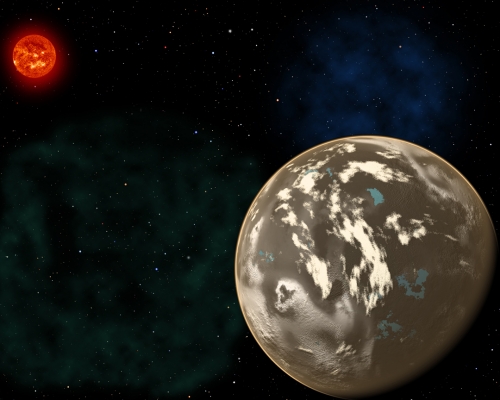
Image: In this artist’s conception, a carbon planet orbits a sunlike star in the early universe. Young planetary systems lacking heavy chemical elements but relatively rich in carbon could form worlds made of graphite, carbides and diamond rather than Earth-like silicate rocks. Blue patches show where water has pooled on the planet’s surface, forming potential habitats for alien life. Credit: Christine Pulliam (CfA). Sun image: NASA/SDO.
Loeb and Mashian point out that the planetary system with the lowest metallicity we’ve yet detected is around the K-class star BD+20 24 57, which shows levels of metals below what was once considered the critical value for planets to form. While CEMP stars are extremely iron-deficient, their carbon abundances make the formation of solid carbon exoplanets a real possibility. Differentiating them from water or silicate worlds could be difficult, but the paper argues that spectral studies of planetary atmospheres could supply the needed markers:
At high temperatures (T ? 1000 K), the absorption spectra of massive (M ? 10 – 60 M?) carbon planets are expected to be dominated by CO, in contrast with the H2O-dominated spectra of hot massive planets with solar-composition atmospheres (Kuchner & Seager 2005). The atmospheres of low-mass (M ? 10 M?) carbon planets are also expected to be differentiable from their solar-composition counterparts due to their abundance of CO and CH4, and lack of oxygen-rich gases like CO2, O2, and O3 (Kuchner & Seager 2005).
So carbon monoxide and methane in the atmosphere could help us tell carbon worlds of similar mass and physical size apart from iron and silicate worlds like the Earth. Detecting carbon planets around ancient stars could provide us with a window into planet formation in the early universe, with implications for where life could form. The paper calls for an observational program using transit methods to search for planets around CEMP stars. Says Mashian:
“This work shows that even stars with a tiny fraction of the carbon in our solar system can host planets. We have good reason to believe that alien life will be carbon-based, like life on Earth, so this also bodes well for the possibility of life in the early universe.”
The paper is Mashian and Loeb, “CEMP stars: possible hosts to carbon planets in the early universe,” accepted at Monthly Notices of the Royal Astronomical Society (preprint).



Just because there is lots of carbon doesn’t imply that this is in any way conducive to the formation of life. Firstly the control of surface temperature might be rather problematic with CO and little metal to allow carbonate rock precipitation. If the deep vent theory of abiogenesis is correct, do hot vents appear with carbon the predominant material? Life as we know it requires Fe, S, P and other rare metals. Can these be substituted away in alien life forms whether their abundances are very much lower?
When searching for life signs on a world that is predominantly reduced carbon, what sort of biosignatures would we look for?
With out iron and nickel the cores of very old planets could be without magnetic fields putting a damper on surface life.
@Alex Tolley. Carbon is also an important element for life but you don’t need a lot of carbon in a planet’s crust to support life but you need some water. The important thing is it’s not the amount of carbon which is important but whether or not you have a carbon cycle working with water. A world like a desert planet with low water might make it difficult for the higher forms of life like animals since the carbon cycle would be limited?
Plate tectonics are assumed to operate on Super Earth and exoplanets larger than Earth. Venus does not have any plate tectonics and such a Super Earths with too much atmosphere or too much water might stop the carbon cycle.http://phys.org/news/2013-11-theoretical-aid-earth-like-planets.html
Imagine a planet without plate tectonics. I am not an expert on geology or planetary science but what happens to the carbon cycle without plate tectonics? The rain takes the Co2 out of the atmosphere and transports it to the ocean through rivers. Through the Urey reaction the soil water combines with Co2 and forms carbonic acid which combines into the calcium silicate to form limestone and chert which travel to the ocean and build up on the sea floor. On a geologic time scale the plates there are subducted below the crust into the subduction zones and mantle to become lava and later out-gassed into the atmosphere as Co2 to keep the carbon cycle in balance.
Without plate tectonics the Co2 is not recycled and the planet beoomes too cold. Wihtout a lot of water there is not a fast enough production of oxygen by life to keep the carbon cycle in balance, so it seems to me that a lot water is very important for life to start and oxygen and ozone production.
The rain eventually takes the Co2 out of the atmosphere but without a lot of rain. The salinity of the small oceans would low as a result. Consequently it might take life longer to form. Exoplanets with carbon rich atmospheres might have a runaway greenhouse effect making it impossible for liquid water or making it scarce.
Carbon-rich earth- and super-earth-mass planets with solid surfaces and medium-pressure atmospheres might actually be rare. Even in systems very enriched in carbon, oxygen abundance is comparable to that of carbon, with C:O ratio somewhere around 2:1 or maybe 3:1. And in a hot reductive carbon-rich environment much of that oxygen would be in form of CO, which probably means 0.05 – 0.3 Earth masses of CO for each Earth mass of the planet itself. So all but the lightest carbon planets should have massive CO envelopes, and if we see a CO-dominated atmosphere on a planet in a carbon-rich system, it is likely an envelope hundreds or thousands of kilometers deep. And no way to measure it, because at multi-GPa pressures CO becomes almost as dense as graphite itself – with only upper hundreds of km less dense than water. But if we see heavier gases (argon?) than it’s probably shallow and a solid graphite surface exists.
There is a theory that before life there was metabolism; specifically, that something like the reductive citric acid cycle (aka rTCA or reverse Kebs cycle) developed under early-Earth conditions, without the polymeric molecules (enzymes) that are associated with it today:
http://www.americanscientist.org/issues/pub/the-origin-of-life/
The funny thing is, the cycle can be supplied and powered by CO alone, rather than CO2 and H2 as is usually depicted:
http://www.ncbi.nlm.nih.gov/pubmed/15596550
If this is true, a carbon planet with a CO/CO2 atmosphere could be ideal for forming primitive life. It could never develop an oxygen atmosphere, though, so things are looking grim for animal life.
Story seed – a group drops off a few species of replicating nanomachines . Fast forward a few centuries; an entire ecology has emerged. The group now visits to collect specimens with promising commercial applications.
A fascinating hypothesis! I want to thank you Paul for your incredible site – I am an avid fan of Centauri Dreams.
Re early stellar generation carbon planets from an exobiology perspective: my major concern is that these low-metallicity planets (literally low in actual metals in this case, I would assume) surely must be even less suitable abodes for life than the higher metallicity, later stellar-generation carbon planets? As the protoplanetary disc cools, methane and water become more stable than carbon monoxide. In lower temperature regimes, carbon monoxide combines with hydrogen via the Fischer-Tropsch reaction to form methane and water initially, later building up longer chain aliphatic hydrocarbons. By itself, the reaction is not very favourable energetically, but it is well catalysed by any iron droplets that have condensed out of the nebula, although even with catalysis, the reaction does not go to completion because the iron droplet surfaces are easily poisoned by iron sulphide or by the F-T reaction products themselves which form gummy carbonaceous tars that coat the iron pellets.
In Solar-type nebulas where the carbon:oxygen ratio is low, only 10% of the nebular material gets converted, so there is abundant hot water vapour to favour the formation of refractory oxides and silicates such as spinel and nepheline and, as the nebula cools further silicates like olivine, feldspars and pyroxenes are formed. Water vapour continues to oxidize metallic iron so Terrestrial silicates contain significant amounts of iron. As solar-type planet formation proceeds, the left over iron and nickel metal sink to the protoplanet center carrying with them most of the sulphides and the cobalt and platinum group metals (because they are denser). The lighter silicates end up floating on the metal base. The end result is a Terrestrial planet with a silicate-rich crust and mantle covering a nickel iron core, with significant amounts of oxidized iron in those same crust and mantle rocks. Some silicates in contact with water form clay minerals, but all are sufficiently oxidized they don’t hydrolyse the water so substantial surface aqueous reserves remain.
In carbon-rich nebulas the situation is very different. In those nebulas, nearly all the available oxygen is bound as carbon monoxide, with little left over for water formation – with the net result being that the overall abundance of water is barely a fifth that of the Solar nebula. Consequently, there is little hot water vapour to react with metallic iron, so very little iron is oxidized; nearly all iron exists as the pure metal or as a sulphate. This process seems to have occurred in isolated pockets in the early Solar nebula where localized high carbon:oxygen ratios created an extreme reducing environment, leading to the formation of enstatite chondrites. In such heavily reducing environments, only the most electronegative metals such as the alkali metals, calcium, aluminium and magnesium, form oxides and silicates. As carbon-rich nebulas cool, the first refractory minerals precipitating out of the cloud are high temperature nitrides and carbides like silicon carbide, titanium nitride and aluminium nitride rather than the more familiar solarian oxides, and the silicates which are formed (largely feldspars and ferromagnesian minerals) are all salted with heavily reduced carbides, nitrides and phosphides. During planet formation nearly all metal (and probably some metallic silicates) will sink to the embryonic core, so effectively iron is chemically fractionated out of the crust and mantle. As on Earth, the lighter silicates (such as sinoite and silicon carbide, which has the same density as olivine) will float on top of the dense iron core forming a thick rocky mantle. Above the silicon and titanium carbide that forms the thick ceramic mantle, a layer of graphite forms, and in a boundary region a score of kilometers thick, under the greatest pressures that same graphite is converted to a layer of diamond.
Water is not thermodynamically stable under these conditions. Even at Earth surface temperatures and pressures silicon carbide will react with water, forming silicon dioxide and liberating hydrogen and carbon, albeit the reaction is sluggish and becomes correspondingly more rapid at higher temperatures (which is significant for subducted strata). Any early planetary oceans in such latter generation carbon planets would be consumed over geologic time, so it seems to me that the Earth mass diamond worlds surely must be completely dessicated (?). Maybe the smaller superterrestrial carbon planets might still have some significant hydrologic reserves?
What could be the saving grace of this early stellar generation carbon planet scenario is the relative scarcity of iron – this means Fischer-Tropsch synthesis would remain relatively unfavoured energetically – unless some of Centauri Dreams’ readers know of some non-metallic, low atomic number catalysts for the F-T reaction? In such a scenario we might easily poison F-T reactions, especially if there were sulphides around.
So – what would we get? A slightly damper carbon world than we would currently get where maybe there are scattered lakes of water (maybe we could even fantasize the occasional shallow, small sea?). Life might evolve – using low atomic number elements – you can use lithium and beryllium, which are reasonable catalysts (after all beryllium chloride is a catalyst for the Friedel-Crafts reaction). Carbon fixation evolving – perhaps, plausibly, by the Monsanto process? Complex oxocarbons playing the role of carbohydrates in terrestrial life? However we view it – such planets are unlikely to have much free metal. The crust would also contain some silicon carbide, crystalline silica, (specifically quartz) and aluminum oxide (predominantly carborundum although this stuff would be very rare). Light metal sulphides such as oldhamite, sinoite and other chemically reduced species -carbides and nitrides- would also be present, but very little iron would be present even if it had been present in appreciable amounts in the early protoplanetary nebula. That raises the question of electrochemical gradients in our hypothetical life – the only possibility I can see, CD’s readers will doubtless come up with better solutions, are the highly eletronegative metals like magnesium, aluminum and the alkali metals, as well as calcium which could be coopted biologically.
Just a few thoughts…. Feedback would be appreciated.
The idea is right – carbon-rich-world chemistry would be profoundly different from oxygen-rich ones’. And details are difficult to investigate because this all is much less usual than the chemistry we’re used to, and no doubt less complex than our own oxidized geochemistry. All the upper right angle of the periodic table – in reduced form, not only halogens and lighter chalcogens – and much of the metals too… And those that are oxidized will be in form of carbides or compounds with other electronegative elements. When I mused about how these worlds could appear, I was startled to realize that some of them might have bodies of liquid sodium on their surface!
Firstly, sodium is abundant. Next, it can be reduced with carbon (Na2CO3 + 2 C -> 2 Na + 3 CO on the planetary scale, or some similar reactions involving sodium compounds without halogens). Then, sodium doesn’t form any stable carbide, unlike almost all other active metals, and it is lighter than graphite – if it is formed by some geochemical process, it would be forced to the surface without being converted into carbide. And there it would stay, especially if the atmosphere doesn’t contain hydrogen. More, sodium is lighter than all it’s salts, even hydride. Indeed it’s unlikely that there would be nothing in the atmosphere and on the surface to react with sodium, but all the products will sink to the bottom. And we will get a planet with shiny metallic ocean – a sodium one! The best conditions are maybe Earth or sub-earth mass, a Mercury-like insolation (so that H2 and excess CO could escape and sodium is molten), and intensive tides to drive internal activity.
Concerning water, it is likely there will be much of the various reducing compounds on the surface that will turn it into H2 and something else, or just chemically bind it. But all the present suggestions about surface chemistry of carbon worlds are a tip of iceberg – even Titan isn’t a good approximation…
PS possibly there is a chance to estimate the probability of sodium-covered worlds – if there is sodium line in the atmosphere of 55 Cancri e, then it’s even more likely these crazy worlds could exist…
My ONLY issue with carbon planets being life generators and life suporters is that the would be ALMOST ENTIRELY LACKING IN WATER! This would lead to the IMPLAUSIBILITY of life AS WE KNOW IT starting up and flourishing. My primary INTEREST in such planets would be life as we DON’T know it. The main question I would have, is: Has improvements in MODELING these planets lead to the conclusion that water CAN be abundant on these planets?
STOP THE PRESSES!!!!! The FIRST bonafide(sorry 55 Cancri e advocates, your planet is PROBABLY a carbon planet, but it is not ABSOLUTELY CERTAIN yet)rocky(i.e. with NO ATMOSPHERE OF ANY KIND)has been detected(but not OFICIALLY DISCOVERED, more on that later), and, to top it all off, shows POSSIBLE evidence of PAST LIFE!!!!!! To anyone who reads this, I suggest you click on http://www.phys.org NOW, read the ENTIRE ARTICLE , and then read the remainder of this comment. Before we pop the champaign, we need ALL OF THE FOLLOWING: ONE, the mass and orbital period of SDSSJ104341.53+085558.2b, so that it can OFFICIALY stand as a planet as opposed to the planet CANDIDATE that it is NOW. TWO: The ISOTOPIC RATIO of the carbon, to determine if the (unconfirmed but MOST LIKELY) limestone was the product of BIOLOGIC ACTIVITY OR NOT. And THREE: A theory that stands up to scrutiny, accounting for ENOUGH WATER on a carbon planet to produce THIS MUCH LIMESTONE IN ITS CRUST!
http://phys.org/news/2016-06-planet-devouring-star-reveals-limestone-crumbs.html
Took ages to find so here’s the direct link
SDSSJ104341.53+085558.2 is NOT the only White Dwarf with calcium in its photosphere. A MUCH YOUNGER(whose PROGINATOR was more massive and luminous than Sirius)White Dwarf with the designation: LP475-242, which has just recently(in astronomical terms, of course)escaped from the Hyades star cluster, whose discovery author claim has NOT JUST ONE KIC8462852-like disintegrating planet, as SDSSJ104341.53+085558.2 APPEARS(not CONFIRMRD, because no TRANSITS have yet been detected)to have, but; instead, AN ENTIRE SYSTEM OF ROCKY PLANETS! It is not clear YET as to whether these putative planets would be Carbon Planets(as SDSSJ104341.53+085558.2b is) should they be CONFIRMED!