Laboratory work on Earth is, as we saw yesterday, leading to hypotheses about how planets form and the effect of these processes on subsequent life. Whether in our own outer Solar System or orbiting other stars, planets in the ‘ice giant’ category, like Uranus and Neptune, remain mysterious, with Voyager 2’s flybys of the latter the only missions that have gone near them. We also know that sub-Neptune planets are common, many of these doubtless sharing the characteristics of their larger namesake.
Thus recent experiments probing ice giant interiors catch my eye this morning. Involving an international team of collaborators, the work looks at the interactions between water and rock that we would expect to find in the extreme conditions inside an ice giant. Planets like Uranus and Neptune are thought to house most of their mass in a deep water layer, a dense fluid overlaying a rocky core, a sharp departure from terrestrial worlds. What happens at that interface is ripe for examination.
The experiments were performed at Arizona State University’s DanShimLab, which is dedicated to the study of planetary materials at a wide range of pressures and temperatures using diamond-anvil and shockwave techniques. To probe this environment, the scientists immersed the rock-forming minerals olivine and ferropericlase in water and then compressed them to high pressures using a diamond-anvil. Heating the sample with a laser, the team could then track the water/mineral reaction under these conditions by way of X-ray measurements.
The result: High concentrations of magnesium, with implications for the composition of oceans much different from Earth’s, as study co-author Sang-Heon Dan Shim (Arizona State University) explains:
“We found that magnesium becomes much more soluble in water at high pressures. In fact, magnesium may become as soluble in the water layers of Uranus and Neptune as salt is in Earth’s ocean. If an early dynamic process enabled a rock-water reaction in these exoplanets, the topmost water layer may be rich in magnesium, possibly affecting the thermal history of the planet.”
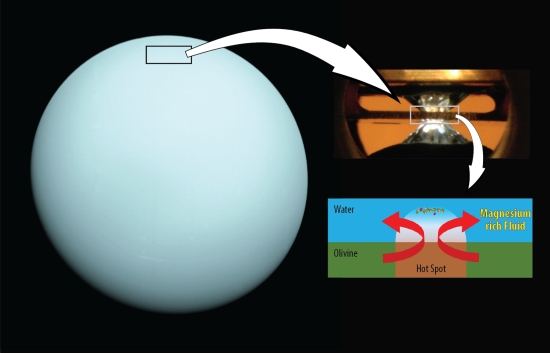
Image: A diamond-anvil (top right) and laser were used in the lab on a sample of olivine to reach the pressure-temperature conditions expected at the top of the water layer beneath the hydrogen atmosphere of Uranus (left). In this experiment, the magnesium in olivine dissolved in the water. Credit: Shim/ASU.
Laser-heated diamond anvil cells can create pressures in the range of 1-5,000,000 bars by compressing materials between diamond ‘anvils’ that are transparent to X-rays, infrared and visible light. The lab’s laser heating systems can take samples to 1,000-5,000 K, all by way of exploring how materials behave under conditions thought to exist in planetary interiors. Thus the range of such laboratory work extends through rocky worlds and into the realm of not just the ice giants but gas giants like Jupiter.
Given the lab’s finding of high concentrations of magnesium under conditions of high pressure and temperature, Shim argues that the mineral may become as soluble in ice giant interiors as salt is in Earth’s oceans. Conceivably, this finding could explain why the atmosphere of Uranus is considerably colder than Neptune’s, for magnesium in larger amounts could block heat from escaping the interior. “This magnesium-rich water may act like a thermal blanket for the interior of the planet,” says Shim.
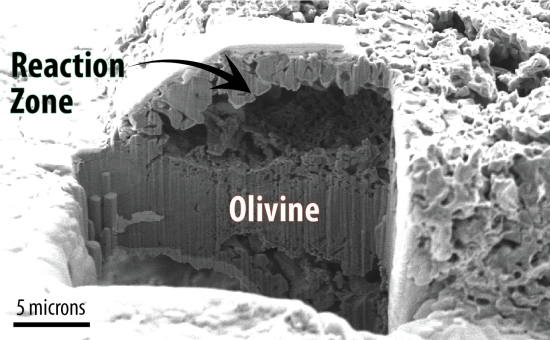
Image: An electron microscopy image of the olivine sample shows a large empty dome structure where magnesium under high-pressure water precipitated as magnesium oxide. Credit: Kim et al.
Oceans rich in magnesium may thus be common in ice giants, with a thick layer of water covering a rocky interior. Moreover, the idea that the interior of water worlds is sharply differentiated between rock and water has been challenged in recent work, with implications for the thermal evolution of these planets. To probe deeper into these matters, the study calls for an examination of other icy materials like CO2 and NH3, but note this cautionary remark in its conclusion:
Extrapolation of our results beyond the pressure range covered in our experiments should [be] treated with caution because of possible changes in the properties of H2O at very high pressures. Nevertheless, the models based on our experiments demonstrate that geochemical cycles and thermal history of water-rich planets could be sensitive to the size of the planet because of pressure-dependent chemical processes.
We have a useful methodology here to extend the study of the geochemical cycle on a range of planetary interiors. We have much to learn, for as the paper points out, the interactions of major rock-forming minerals at the interface between ocean and rock in ice giants have rarely been explored at the high pressures used in these experiments.
The paper is Kim et al., “Atomic-scale mixing between MgO and H2O in the deep interiors of water-rich planets,” Nature Astronomy 17 May 2021 (abstract).



Could this explain why our Sol’s Ice Giants have magnetic fields which are “wonky”?
No. The magnetic fields are caused by the motion of pressure ionized water ammonia. Also I recall reading that another idea to explain Uranus lack of detectable internal heat radiation is the collision that knocked Uranus on its side with a nearly right angle axial tilt which caused it to loose it’s internal heat. Wikipedia, Uranus.
“…This magnesium-rich water may act like a thermal blanket for the interior of the planet…”
Ummm…… OK.
That heat still must escape even if slowed down by some blanket. And after equilibrium…… lets just say 100 millions years…. atmosphere top release should approach the core release thermal.
Nature continues to demonstrate that complex conditions are common, defying simple initial thoughts on conditions. If magnesium-rich liquids (or water ices) exist in ice giants, this may be yet another resource that a future space-faring civilization might extract and use.
Is it selection bias, or is there more research appearing that indicates that we need to take more phenomena into account to explain the details of celestial bodies in our solar system, including Earth?
Appearing since when? Discoveries indicating the Solar System and its origins are more complex than previously thought have been accumulating since…well…since the first exoplanet detections, at minimum.
I’d be tempted to say “since Voyager” but I’m not sure the Voyager data had anything that challenged the static model I remember from school.
I note as well that with our four solar system gas giants, the bluish hue of the outer two are supposedly attributed to concentrations of methane in the outer atmosphere. This also raises as many questions as it answers.
There is a substantial amount of methane in Titan’s atmosphere. And also, the abundances of methane associated with Uranus and Neptune indicate a lower abundance of hydrogen and helium, if interpreted another way.
I can’t think of a good reason why hydrogen and helium should escape that much more easily for these two planets, arranged as they are so far from the sun, but perhaps it is an argument for an early re-arrangement of the solar system as the Nice group argues for, migrations that look more like castling on the chess board.
But going back to the Neptune sized planets as a category, once some
spectral imaging of exoplanets of the same mass are obtained, contrasting the bluish hue and the methane lines should be an interesting experiment.
thanks, Geoffrey.
But you would think a big collision would heat up a planet?
For those interested the full text of the study’s preprint is currently available here: https://www.researchsquare.com/article/rs-78494/v1
It would heat up a planet, but only temporarily I’m not sure what the exact physics of the idea of Wikipedia, but I think it has to do with the core size. Uranus core is .55 or about half an Earth mass which half the size of Neptune’s larger core of 1.2 Earth masses which I assume they mean Uranus core got smaller from the giant impact which in theory could explain the lower heat. There still is some heat due to the compression of gravity, but Uranus does not radiate as much heat as Neptune. Source Wikipedia, Uranus and Neptune.