A kilonova at the wrong place and time would spell trouble for any lifeforms emerging on a planetary surface. Just how we found out about kilonovae and the conditions that create them, not to mention their hypothesized effects, is the subject of Don Wilkins’ latest, a look at Cold War era surveillance that wound up pushing astronomy’s frontiers. That work now causes us to ponder the formation of an ‘island of stability’ in which exists a set of superheavy element isotopes with unique properties. It also raises interesting questions about our Solar System’s history and possible exposure to a nearby event. Based at Washington University in St. Louis, Don’s interest in deep space exploration here probes the formation and structure of matter in processes we’re only beginning to unlock.
by Don Wilkins
Setting out to discover something on Earth can sometimes reveal an unexpected result from a far more interesting source. As a case in point, consider what happened in August of 1963, when Great Britain, the US and the USSR signed a nuclear test ban treaty forbidding nuclear detonations in space or the Earth’s atmosphere. For the older space nerds, this is the same treaty that ended the Orion program. Given the Soviets’ history of violating treaties, the US launched the Vela (derived from the Spanish verb “velar”, to watch) series of satellites designed to monitor compliance with the treaty within two months of the signing. What they found was a bit of a surprise.
The satellites were heavily instrumented with x-ray, gamma-ray, neutron, optical and electromagnetic pulse (EMP) detectors along with other sensors designed to monitor the space environment. The satellites operated in pairs on opposite sides of a circular 250,000 kilometers in diameter orbit, Figure 1.
Figure 1. A Pair of Vela Satellites Readying for Launch. Los Angeles Air Force Base, U.S. Air Force Photo.
X-ray detectors directly sense nuclear blast. Gamma-ray and neutron detector activations would confirm the nuclear event and would prompt a stiffly worded diplomatic note sent to the Soviets. Vela satellites were positioned to monitor the Earth and the far side of the Moon. The latter involved detecting gamma radiation from radioactive debris scattered by a clandestine explosion. As a result of the separation of the satellites and separation in time between sensor triggering on the satellites, the angle to the event could be determined to about one-fifth of a radian or ten degrees. Angles to a single event observed by multiple pairs of satellites could provide a more precise direction to the source.
No diplomatic note concerning illegal nuclear tests was ever sent to the Soviets. Fortunately events which triggered the detectors but were clearly not signatures of nuclear detonations were not discarded. These formed a database which eventually led to the discovery of enormous, but short-lived gamma-ray bursts (GRBs) originating in deep space. GRBs last less than three seconds (although a recent discovery lasted an astounding 200 seconds), yet they are as luminous as 100 million galaxies, the equivalent of a 1000 novae. Gamma-ray sources have temperatures of approximately 109 K degrees and are among the hottest objects ever observed. Compounding the mystery, researchers only had a line pointing to the origin of the bursts but no distance.
GRBs occur daily and are uniformly distributed across the observable Universe. Initially no counterpart of the GRBs operating in the visual spectrum could be found. Then, in 1997, Italian astronomers caught the fading light of an object which could be linked with a GRB, Figure 2.
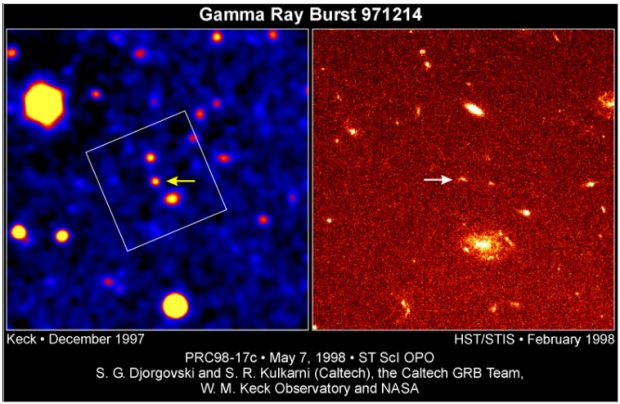
Figure 2. Left: Arrow points at the GRB optical counterpart. Right: An IR image of the tilted box area in the left image. The optical source is gone, and only a faint image of a very distant galaxy remains. The other two bright sources on the right side are spiral galaxies. Credit: W. M. Keck Observatory / NASA.
The favored explanation for GRBs is the collision of two neutron stars or two black holes. Astronomers named the neutron star mergers kilonovae (KN). In addition to GRBs, these collisions emit high-frequency gravitational waves (GW) and are, through rapid neutron capture (the r-process) nucleosynthesis, likely production sites of heavy elements. [1] A team led by Andres Levan examined spectroscopy of GRB 230307A, a long-duration GRB associated with a kilonova merger. A 2.15 micron emission line from that analysis is associated with tellurium (atomic mass 130), and a mid-IR peak, lanthanides production. GRB nucleosynthesis creates a wide range of atomic masses including heavy elements (mass above iron). [2]
These observations and others support the hypothesis that heavy elements within the Solar System are the remnants of a kilonova.[3-4]
Figure 3 depicts the evolution of a neutron star merger over the course of millennia. The drawing on the left depicts the aftermath a few years after the merger and at dimensions below a parsec. Gamma-rays are emitted in the dynamic ejecta and the hot cocoon. The gamma-ray jet and cocoon emissions are short-lived; the afterglow they produce emits broadband frequencies for several years. The dynamic ejecta include heavy elements which decay in less than a month to produce the UV, optical and IR displays. X-ray emissions, at potentially lethal levels, result from the interaction between the jet and the interstellar medium (ISM).
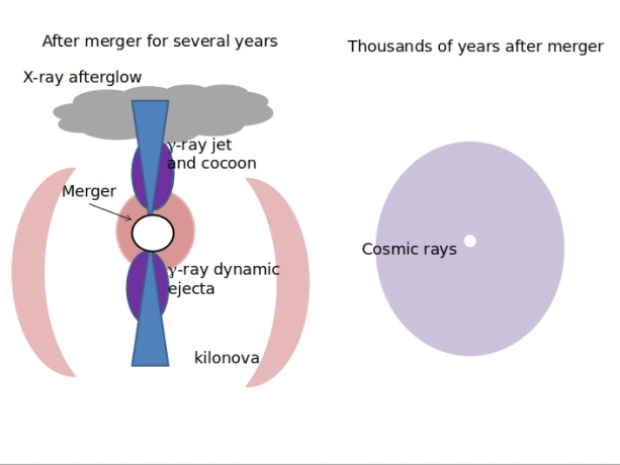
Figure 3. Structures Resulting from Neutron Star Merger
On the right hand side, a powerful shock wave from the merger produces a bubble in the ISM. Potentially lethal cosmic rays result.
Initial analysis of GRBs focused on the on-axis gamma ray bursts. M.L. Perkins’ team analyzed the data to understand threats by the off-axis emissions and the relation to other cosmic threats. [5]
According to the team:
For baseline kilonova parameters, … the X-ray emission from the afterglow may be lethal out to ∼ 5 pc and the off-axis gamma-ray emission may threaten a range out to ∼ 4 pc, whereas the greatest threat comes years after the explosion, from the cosmic rays accelerated by the kilonova blast, which can be lethal out to distances up to ∼ 11 pc. … . Based on the frequency and potential damage done, the threats in order of most to least harmful are: solar flares, impactors, supernovae, on-axis GRBs, and lastly off-axis BNS mergers.
One question concerns how close to Earth a kilonova may have manifested. The presence of two isotopes, iron-60 (Fe-60) and plutonium-244 (Pu-244) found in ocean sediments deposited 3 to 4 million years ago offers clues. These isotopes are only formed in very energetic processes.
Fe-60 can, in theory, be created in a standard supernova. Pu-244 is created only in specific classes of supernovae or the merger of a neutron star with another astronomical body, the kilonova.

Figure 4. Artist’s impression of a neutron star merger. Credit: University of Warwick / Mark Garlick.
One of the problems was the ratio between the isotopes. Researchers at the Università di Trento found, with a specific debris ejection pattern and a certain tilt of the merger event, the observed ratio of iron to plutonium isotopes could be explained by a kilonova. [6] The scientists examined rare types of supernovae such as a magneto-rotational supernova or collapsar, but concluded the kilonova was the source of the isotopes.
To determine how far from Earth the kilonova occurred, the researchers calculated the different spreads for each element based on the wind speed created by the kilonova. The answer was about 150 to 200 parsecs or about 500 to 600 light years away.
Hydrogen and helium were created with the Big Bang; heavier elements were made by fusion within the interior of stars, supernovae and kilonovae. Data provided by astronomer Jennifer Johnson from Ohio State University was used to produce the periodic table depicting the origins of elements shown in Figure 5 below.
Researchers have examined the heavy element composition of a number of stars, finding that some of these elements are the product of the radioactive decay of previously unobserved elements. [7] These predecessor elements form in a theorized “island of stability” with atomic numbers centered around 126. Isotopes in this region, beyond the fleeting transuranics, are hypothesized to possess “magic numbers” of protons and neutrons that allow them lifespans of thousands or millions of years. The rapid neutron-capture process that occurs in neutron-rich environments of neutron star mergers and supernovae appears inadequate to form the elements in the island of stability. How these transuranics were produced is a mystery.
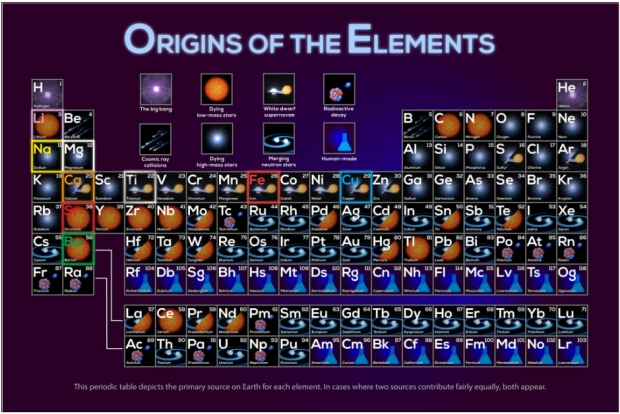
Figure 5. Origins of Elements – Courtesy NASA’s Goddard Space Flight Center.
The effects of neutron star mergers, like rain, depends on timing. In the early stages of star formation, the collisions shower the clouds of hydrogen and helium with heavy metals necessary for life. Yet after life gained its foothold, an improperly timed – and ill-placed – kilonova could severely damage or erase what a predecessor started.
References
1. B. D. Metzger, G. Martínez-Pinedo, S. Darbha, E. Quataert, A. Arcones, D. Kasen, R. Thomas, P. Nugent, I. V. Panov, N. T. Zinner, “Electromagnetic counterparts of compact object mergers powered by the radioactive decay of r-process nuclei,” Monthly Notices of the Royal Astronomical Society, Volume 406, Issue 4, August 2010, Pages 2650–2662, https://doi.org/10.1111/j.1365-2966.2010.16864.x
2. Levan, A., Gompertz, B.P., Salafia, O.S. et al. “Heavy element production in a compact object merger observed by JWST.” Nature (2023). https://doi.org/10.1038/s41586-023-06759-1
3. Bartos, I., Marka, S. “A nearby neutron-star merger explains the actinide abundances in the early Solar System.” Nature 569, 85–88 (2019). https://doi.org/10.1038/s41586-019-1113-7
4. Watson, Darach, Hansen, Camilla J., Selsing, Jonatan, et al, “Identification of strontium in the merger of two neutron stars,’ arXiv:1910.10510 [astro-ph.HE], 23 Oct 2019
5. Perkins, M.L., Ellis, John, Fields, B.D, et al, “Could a Kilonova Kill: a Threat Assessment,” arXiv:2310.11627v1, 17 October 2023.
6. Leonardo Chiesa, et al, “Did a kilonova set off in our Galactic backyard 3.5 Myr ago?,” arXiv (2023). DOI: 10.48550/arxiv.2311.17159
7. Ian U. Roederer, et al, “Element abundance patterns in stars indicate fission of nuclei heavier than uranium,” Science, 7 Dec 2023, Vol 382, Issue 6675, pp. 1177-1180, DOI: 10.1126/science.adf1341



Speaking of Project Orion…
https://www.centauri-dreams.org/2016/09/16/project-orion-a-nuclear-bomb-and-rocket-all-in-one/
If there are elements in the “island of stability” that are both stable long enough, as well as needed for some elements to be created, why is there apparently no evidence of them? Shouldn’t these elements show up with very low frequencies using mass spec, and in the spectra around the stars that formed them? Or they are in some cosmic rays, could we not detect them directly?
IOW, if as indicated in your reference 7, the pattern of elements infers the prior presence of these superheavy elements, why is there no evidence of them in nature? Or is there?
I understand the spectra for transuranics when heated to stellar temperatures are not well understood. Another complication is the purported sightings of transuranic spectrum are in F stars. These stars have a high overall rotation and various parts of the star rotate at rates. These further complicate taking spectra.
Was it the Vela satellites, or different ones, that detected a possible nuclear explosion in or near S. Africa decades ago?
It was (although the detection was only in visible light, which makes sense given that gamma rays and X-rays would be blocked by the atmosphere).
https://en.wikipedia.org/wiki/Vela_incident
I could do better commentary if there were a good free online dilettante-level QCD course to study. :) The paper itself ( https://arxiv.org/ftp/arxiv/papers/2312/2312.06844.pdf ) is notably laconic in interpretations, beyond the bare statement that atoms with mass > 260 seem to be involved by their model (which is general and statistical, and doesn’t indict an isotope of unobtainium and indeed may not need it). I wonder whether such isotopes could be formed before the kilonova in the unknown depths of neutron stars. But the details there get peculiar: for example, the Wikipedia article on neutron stars raises the point that neutron-rich elements could be stabilized by the pressure, and that’s just the start of it. Deeper in, there might be “nuclear pasta” that comes in spaghetti, lasagna, bucatini, and Swiss cheese, and below that neutron-degenerate matter, and below that a color superconductor or even a quark-gluon plasma. There’s a gloriously overwhelming crash course in the physics at http://cds.cern.ch/record/1443909/files/CERN-2014-001.pdf (see page 219 for the phase diagram).
Neutron star mergers seem like a fun untapped opportunity for SETI to try to see if FTL is possible. Suppose an intelligent life form arose prior to hadronization, in the first microsecond after the Big Bang. With the Hagedorn temperature being near 1.7 trillion K or 160 Mev, I’d assume “reactions” in quark-gluon plasma happen far faster than in conventional matter. Comparing the time scales to evolution, a quark matter civilization that somehow found a way to survive until the formation of the first neutron stars, or evolved in the core of a neutron star later, might be something like an octillion, in human years. Supposing they chose to remain short of Kardashev II, they would need to flee neutron star mergers, presumably by resettling on new neutron stars. The process of QCD-a-forming the new star and moving their entire culture seems daunting enough that it might take hours and be visible to human astronomers. Hence: if a civilization on this level of matter exists, a supernova leading to the birth of a neutron star may be *preceded* by a burst of released energy on nearby neutron stars, especially doomed ones, with proper timing to allow work teams to arrive at near lightspeed as the cool (0.1 terakelvin) core of the supernova begins its collapse. Unless FTL exists apart from time travel in the general sense, in which case some spacelike reference surface should relate events on the neutron stars instead.
Just a quick note to say that that resolution for the figures in the articles is often too low. I was interested in the “Origins of the elements” figures but I can’t read what the symbols mean, even when saving it and zooming it.
Enzo, here’s a link to the original image (actually two of them), which should be better:
https://svs.gsfc.nasa.gov/13873
The physics of the red color of barns
We should be so lucky that all of the elements than can be made only through neutron star mergers are not used in biology. Compared to novae or supernovae, I would imagine that neutron star mergers are relatively rare. It would be a shame if some of the trace elements needed for biology could only be made in such a manner. Because if so, there would be major regions of the galaxy that would not only be devoid of life, but that life could not even settle there without the ability to transmutate elements on an industrial scale.
I think we’re almost that lucky, with two exceptions I can think of. Molybdenum has an ancient role in the function of a handful of key enzymes, but judging by the higher-res graphic at Wikipedia’s “r-process” (sorry…), less than half is produced by r-process. But then there is iodine, uncommon and nearly all of which is produced by the r-process. The bad news is that thyroid hormone regulation is spread widely among animal phyla, but the good news is that some groups have already evolved away from depending on it. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10083991/ The hormone is truly the worst Rube Goldberg ‘unintelligent design’ I can think of in biology – people are dependent on a trace nutrient that historically was often unavailable in local diets, in order to produce large amounts of a big protein in a special gland merely to rearrange a few of the tyrosine amino acids into a new chemical. My feeling is that this is likely to be fixed long in advance of interstellar colonization, because the mechanism also accumulates radioactive iodine from fallout. Beginning first in agriculture, swapping out T3 and T4 regulation for insect ecdysteroids should help to make livestock less radioactive. It would be accepted even to break the regulatory pathways, so long as the livestock grow bigger on average. The biology is complex and difficult to get completely right, but eliminating human iodine uptake would be an urgent priority after widespread nuclear war, and there ought to be a way to do it. Once this one use is eliminated, the need for iodine in the diet is at least not currently obvious.
It would appear that iodine is used by almost all life forms suggesting that it is hard to substitute for in terrestrial biology.
Iodine in biology
Recall that phosphorus is often a limiting element in biology, but there is no substitute in energy production and RNA and DNA structure.
Looking at that link and https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9182735/ I don’t see very much detail or confirmation regarding other biological roles of iodine. Some of the phenomena mentioned are consequences of having the thyroid hormone system: concentration in milk, apoptosis of frog tails. The antioxidant activity is real but surely not unique to this element. The anticancer role is interesting, but at least is not being widely pursued in current treatment of cancer patients and people at risk. I found an old paper about iodine ending up in a protein complex at the skin in Drosophila ( https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1079057/ ) but I don’t know if it is important.
I should admit confusion regarding the livestock: I recalled the Saami people had been affected by radioactive iodine after Chernobyl, but it was only a short-lived isotope (I-131) and now the culprit is radioactive cesium-137. ( https://engagingvulnerability.se/wp-content/uploads/2016/03/1990_HSBpercepchernOCR.pdf ) Unless there were continuing ongoing nuclear explosions the iodine project would be on a back burner. It ought to be much easier to CRISPR a microbial non-selective Cs+/K+ leak channel for intestinal expression in reindeer, but alas, nobody ever did that much.
there would be major regions of the galaxy that would not only be devoid of life
Why do you say this? Neutron-star mergers would happen pretty much anywhere in the galaxy, and their products would gradually diffuse throughout the interstellar medium and thus available for future star and planet formation (as is the case for the products of supernovae).
They can. But very few stars are big enough to nova into neutron stars. There are even fewer of these stars that exist in pairs or triads.
I took this as a reference to the Milky Way’s stellar halo, whose stars tend to be metal-poor overall. They are also more widely dispersed, so have fewer chances to pass near a former neutron star merger. The halo has a large physical volume and presumably great importance for any slow mechanism of intergalactic colonization.
Sorry I’m late on this, but in reference to the previous thread “Close Stellar Encounters and Earth’s Orbit”, I have added a quick aid to calculating those encounters. Please refer to that thread for the new post.