Energetics of Archaean Life in the Ocean Vents
If SETI is all about intelligence, and specifically technology, at the other end of astrobiology is the question of abiogenesis. Does life of any kind in fact occur elsewhere, or does Earth occupy a unique space in the scheme of things? Alex Tolley looks today at one venue where life may evolve, deep inside planetary crusts, with implications that include what we may find “locally” at places like Europa or Titan. In doing so, he takes a deep dive into a new paper from Jeffrey Dick and Everett Shock, while going on to speculate on broader questions forced by life’s emergence. Organisms appearing in the kind of regions we are discussing today would doubtless be undetectable by our telescopes, but with favorable energetics, deep ocean floors may spawn abundant life outside the conventional habitable zone, just as they have done within our own ‘goldilocks’ world.
by Alex Tolley

Are the deep hot ocean vents more suitable for life than previously thought?
In a previous article [1] I explored the possibility that while we think of hot planetary cores, and tidal heating of icy moons, as the driver to maintain liquid water and potentially support chemotrophic life at the crust-ocean interface, radiolysis can also provide the means to do the same and allow life to exist at depth in the crust despite the most hostile of surface conditions. On Earth we have the evidence that there is a lithospheric biosphere that extends to a depth of over 1 kilometer, and the geothermal gradient suggests that extremophiles could live several kilometers down in the crust.
Scientists are actively searching for biosignatures in the crust of Mars, away from the UV, radiation, and toxic conditions on the surface examined by previous landers and rovers. Plans are also being drawn up to look for biosignatures in Jupiter’s icy moon Europa, where hot vents at the bottom of a subsurface ocean could host life. It is hypothesized that Titan may have liquid water at depth below its hydrocarbon surface, and even frozen Pluto may have liquid water deep below its surface of frozen gases. The dwarf planet Ceres also may have a slushy, salty ocean beneath its surface as salts left by cryovolcanism indicate. Conditions conducive to supporting life may be common once we look beyond the surface conditions, and therefore subsurface biospheres might be more common than our terrestrial one.

Image: Rainbow vent field. Credit: Royal Netherlands Institute for Sea Research.
The conditions of heat and ionizing radiation at depth, coupled with the appropriate geology, and water, are energetically favorable to split hydrogen (H2) from water, and then reduce carbon dioxide (CO2) to methane (CH4) via the serpentinization reaction. Chemotrophs feed on this reduced carbon as fuel to power their metabolisms. This reaction has an energy barrier that results in more reactants than products than would be expected at equilibrium. As the reaction energetics are favorable, life also evolves to exploit those reactions, with catalytic metabolic pathways that overcome the energy barrier and allow the equilibrium to be reached, realising the reaction energy..
Biologists now classify life into 3 domains: bacteria, eukaryotes, and the archaea. The bacteria are an extremely diverse group that represent the most species on Earth. They can transfer genes between species, allowing for rapid evolution and adaptation to conditions. [It is this horizontal gene transfer that can create antibiotic resistance in bacteria never previously exposed to these treatments.] The eukaryotes, which include the plants, animals and fungi, range from the single cell organisms such as yeast and photosynthetic cyanobacteria, to complex organisms including all the main animal phyla from spongers to vertebrates. The archaea were only relatively recently (1977) recognized as a distinct domain, separate from the bacteria. Archaea include many of the extremophiles, but perhaps most importantly, exploit the reduction of CO2 with H2 to produce CH4. These archaea are called autotrophic methanogens and require anaerobic conditions. The CH4 is released into the environment, just as plants release oxygen (O2) from photosynthesis. In close proximity to the hot, reducing ocean vent conditions, cold, oxygenated seawater supports aerobic metabolisms, resulting in a biologically rich ecosystem, despite the almost lightless conditions in the abyssal ocean depths.
While CH4 and other reduced carbon compounds are both abiotically and biotically produced, we tend to assume that the formation of biological compounds such as amino acids requires energy that is released from the metabolism of the fixed carbon from autotrophs, whether CH4, or sugars and fats by complex organisms. While this is the case in the temperate conditions at the Earth’s surface, metabolic energy inputs do not appear to be needed under some ocean vent conditions.
The energetics of principally amino acids and protein synthesis is explored in a new paper by collaborators Jeffrey Dick and Everett Shock [2], building on their prior work. The paper examines conditions at two vent fields, Rainbow and Endeavour, compares the energetics of amino acids in those locations, and relates their findings to the proteins of the biota. The two vent fields have very different geologies. The Rainbow vent field is located on the Mid-Atlantic Ridge, at the Azores, and is composed of ultramafic mantle rock that is extruded to drive apart the tectonic plates, slowly widening the Atlantic ocean. In contrast, the Endeavour vent field is located in the eastern Pacific ocean, southwest of Canada’s British Columbia province, and is part of the Juan de Fuca Ridge. It is principally composed of the volcanic mafic rock basalt.
Mafic rocks such as basalt have a silica (SiO2) content of 45-53% with smaller fractions of ferrous oxide, alumina, calcium oxide, and magnesium oxide, while ultramafic peridotites such as olivine have a SiO2 content below 45%, and are mainly comprised of magnesium, ferrous silicate [(Mg, Fe)SiO4]. As a result of the difference in composition and structure, ultramafic rocks produce more hydrogen than the higher SiO2 content mafic rocks.
Typically, the iron sequesters the O2 from the serpentinization reaction to form magnetite (Fe3O4), preventing the H2 and CH4 from being oxidized. The authors use the chemical affinity measure, Ar, to explore the energetic favorability of the production of CH4, amino acids, and proteins. The chemical affinities are positive if the Gibbs free energy releases energy in the reaction, and the reaction is kept further from completing the reaction to equilibrium; that is more reactants and less product than the equilibrium would indicate. Positive chemical affinities indicate that there is energy to be gained from the reaction reaching equilibrium.
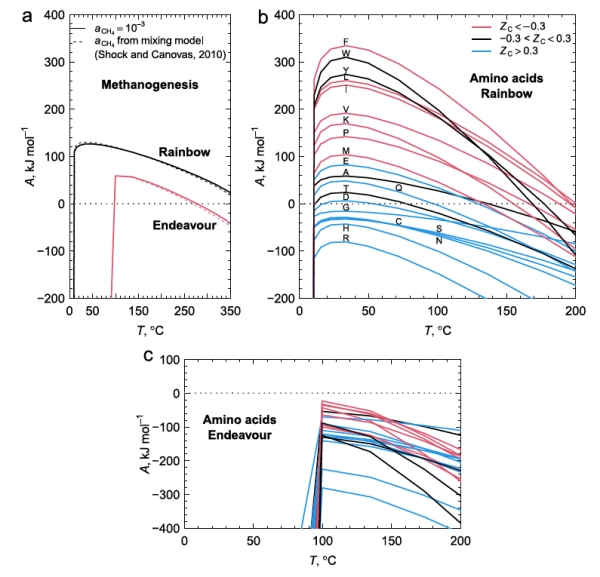
Figure 2 below shows the calculated chemical affinity values for a range of temperatures at the two ocean vent fields Rainbow and Endeavour, at different temperatures as a result of the hot vent water mixing with the cold surrounding seawater. They show that the ultramafic geology at Rainbow has positive affinities for both CH4 and most amino acids, while Endeavour has positive, but lower, affinities for CH4, but negative affinities for amino acids. The Endeavour field not only has lower CH4 affinities for any temperature compared to Rainbow, but this field also has a positive affinity cutoff temperature at about 100C, well above that of Rainbow. As few organisms can live above this temperature, this indicates that methanogens living at Endeavour cannot use the potential free energy of CH4 synthesis to power their metabolisms.
Figure 2b shows that the peak affinities for the amino acids at Rainbow are at around 30-40C, similar to that of CH4. While the range of temperatures where most amino acids have positive affinities at Rainbow to allow organisms to gain from amino acid synthesis, the conditions at Endeavour exclude this possibility in its entirety. As a result, Rainbow vents have conditions that life can exploit to extract energy from amino acid, and hence protein production, whilst this is not available to organisms at Endeavour.
Exploitation of these affinities by life at these two vent fields indicates that autotrophic methanogens will only likely gain metabolic energy from producing CH4 and from anabolic metabolism to produce many amino acids at Rainbow, but not at Endeavour. This would suggest that the Rainbow environment is more conducive to the growth of methanogens, whilst Endeavour offers little competitive advantage against chemotrophs.
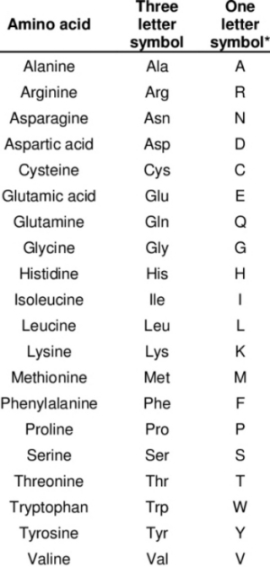
Figure 1. The 20 amino acids and their letter codes needed to interpret figure 2b.
Figure 2. a. CH4 production releases more energy at the Rainbow hot vent field with ultramafic geology compared to the mafic Endeavour field when the hot fluids at the event are mixed with cold 2C seawater in greater amounts to reduce the temperature. b. The energetics of amino acid formation at Rainbow. More than half the amino acids are energetically favored. c. All amino acids are not energetically favored at Endeavour primarily due to the much lower molar H2 concentrations at Endeavour.
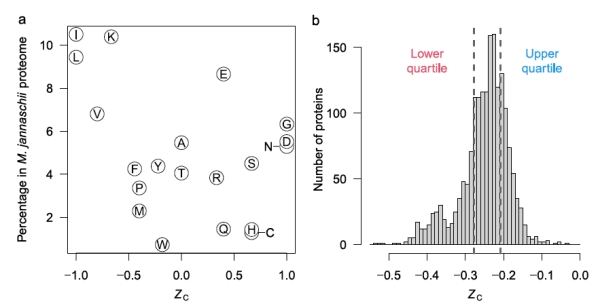
Figure 2b shows that some amino acids release energy when hot 350C water with reactants from Rainbow vents is mixed with cold seawater (approximately 6-10x dilution), while others require energy. The low H2 concentration in samples from Endeavour vents, about 25x more dilute, accounts for the negative affinities across all mixing temperatures at Endeavour. Why might this difference in the affinities between amino acids exist? One explanation is shown in figure 3a, that shows the oxidation values (Zc) of the amino acids. [Zc is a function of the oxidizing elements, charge, and is normalized by the number of carbon atoms of each amino acid. This sets a range of values as [-1.0,1.0].] Notably, those more energetically favored in figure 2b are also those that tend to be least oxidized, that is, they are mostly non-polar, hydrophobic amino acids with C-H bonds dominating.
Figure 3. a. The oxidation level of amino acids. The higher the Zc value, the greater the number of oxidizing and polar atoms composing the amino acid. b. Histogram of all the proteins in the archaean Methanocaldococcus jannaschii based on their per amino acid carbons oxidation score.
Figure 3b shows the distribution of the Zc scores for the proteins of the archaean Methanocaldococcus jannaschii that is found in samples from Rainbow field. The distribution is notably skewed towards the more reduced proteins. The authors imply that this may be associated with the amino acids that have higher affinities and therefore their energy release of formation can be exploited by M. jannaschii.
The paper shows that all the organism’s proteins with their varying amino acid sequences have positive affinities from 0C to nearly 100C. As M. jannaschii has a preferred growth temperature of 85C, its whole protein production produces a net energy gain rather than requiring energy at this vent field, but would not have this energetic advantage if living at Endeavour. While M. jannaschii has an optimum growth temperature of 85C, one might expect other methanogens with optimal growth at lower temperatures closer to those of the optimal affinity values would have a competitive advantage.
As the authors state:
“Keeping in mind that temperature and composition are explicitly linked, these results show that the conditions generated during fluid mixing at ultramafic-hosted submarine hydrothermal systems are highly conducive to the formation of all of the proteins used by M. jannaschii.”
As the archaea already exploit the energetics of methane formation, do they also exploit the favorability of certain amino acids in the composition of their proteins, which are also favored energetically as the peptide bonds are formed?
While figure 3b is an interesting observation for one archaean species found at Rainbow, a natural question to ask is whether the different energetic favorability of certain amino acids is exploited by organisms at the vents by biasing the amino acid sequence of their proteomes, or whether this distribution is common across similar organisms both hot vent-living and surface-living organisms of methanogen archaea and other types of bacteria.
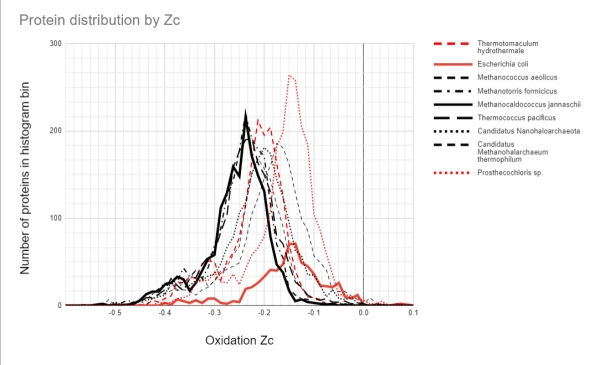
To put the M. jannaschii proteome Zc distribution in context, I have extended the authors’ analysis to other archaea and bacteria, living in hot vents, hypersaline, and constant mild temperature environments. Figure 4 shows the proteome Zc score distribution for 9 organisms. The black distributions are for the archaeans, and the red distributions for bacteria. The distributions for M. jannaschii and the model gut-living bacterium Escherichia coli are bolded.
Figure 4. Histogram of proteome oxidation for various archaea (black) and bacteria (red). Several archaea living in hot temperatures are clustered together. The anaerobic, gut-living E. coli has a very different distribution. The bacterium Prosthecochloris that also lives in the hot vents has a distribution more similar to E. coli, whilst the hot vent living T. hydrothermale has a distribution between the vent-living archaea and E. coli. Two of the archaea also have distributions that deviate from the vent archaeans, one of which is adapted to the hot, hypersaline volcanic pools on the surface. (source author, Alex Tolley)
Figure 4 suggests that the explanation is more complex than simply the energetics as reflected in the proteome’s amino acid composition.
Firstly, the proteome distributions of M. jannaschii and E. coli are very different. They represent different domains of life, inhabit very different environments, and only M. jannaschii is a methanogen. So we have a number of different variables to consider.
Several archaea, all methanogens living in vents at different preferred temperatures, have similar proteome Zc score distributions. The two hypersaline archaea, Canditatus sp., have their distributions biased towards higher Zc scores that may reflect proteomes that are evolved to handle high salt concentrations. One is likely a methanogen, yet its distribution is further biased to a higher Zc score than the other. Of the bacteria, the hot vent-living Thermotomaculum hydrothermale has a Zc score distribution between E. coli and the similar archaean group. It is not a methanogen, but possibly it exploits the amino acid affinities of the hot vent environment.
The other vent-living bacterium, Prosthecochloris sp., has a distribution like that of E. coli. It is a photosynthesizing bacteria similar to green sulfur bacteria, and extracts geothermal light energy. It is not a methanogen. It is found in the sulfur rich smoker vents of the East Pacific Rise.
There seems to be two main possible explanations for the proteomic Zc distributions. Firstly, it may be due to a bias in the selection of amino acids that could release energy when in the H2-rich Rainbow habitat. Secondly, it could be the types of protein secondary structures that are needed for methanogenesis, so that structural reasons are the cause.
Figure 5 shows the protein structures for three approximately matching Zc scores and sequence length for M. jannaschii and E. coli. What stands out is that the lower the Zc score, the more the alpha-helix secondary structure appears in the protein tertiary structure. Both organisms appear to have similar secondary structure compositions when the Zc scores are matched, suggesting that the distribution differences are due to the numbers of proteins with alpha-helix structures rather than some fundamental difference in the sequences. Is this a clue to the underlying distribution?
The amino acids that principally appear in helices are the “MALEK” set, methionine, alanine, leucine, glutamic acid and lysine [6]. A helix made up of equal amounts of each of these amino acids has a Zc score of -0.4, all coincidently in the positive affinity range of amino acids in Rainbow, as shown in figure 2b. This is highly suggestive that the reason for the different distributions is a bias in the production of proteins that have an abundance of alpha-helix secondary structures.
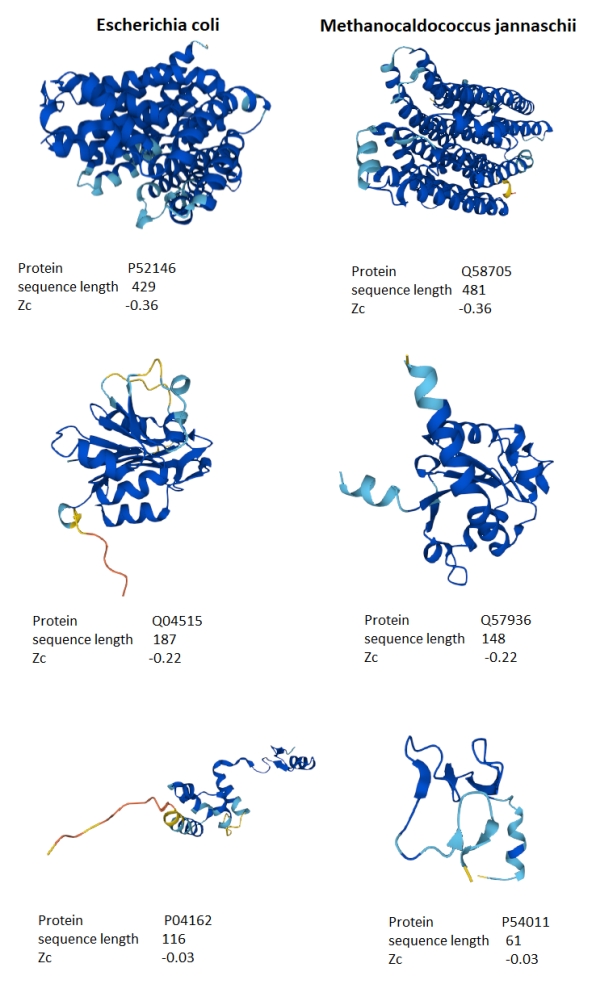
Figure 5. Comparison of selected proteins from M. jannaschii and E. coli spanning the range of Zc scores.
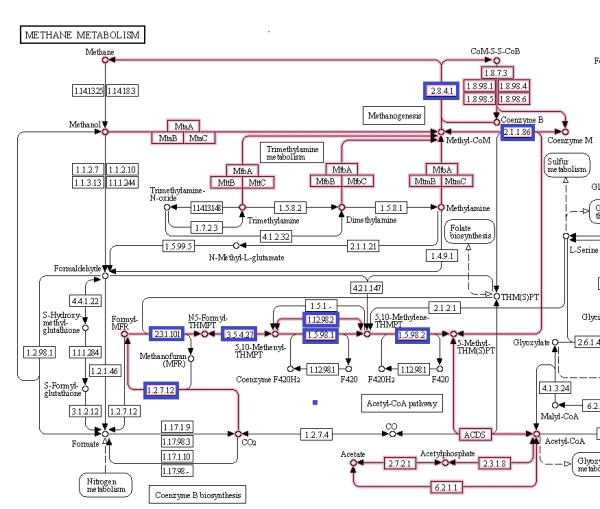
Which proteins might be those with sequences that have higher high alpha-helix structures? As a distinguishing feature of archaea is methanogenesis, a good start is to look at the proteins involved in methane metabolism.
Figure 6 shows the methanogenesis pathways of methane metabolism highlighted. The genes associated with the methanogenesis annotated proteins of M. jannaschii are boxed in blue and are mostly connected with the early CO2 metabolism. From this, some proteins were selected that had tertiary structure available to be viewed in the Uniprot database [3].
Figure 6. Methane metabolism pathway highlighted. Source: Kegg database [4].
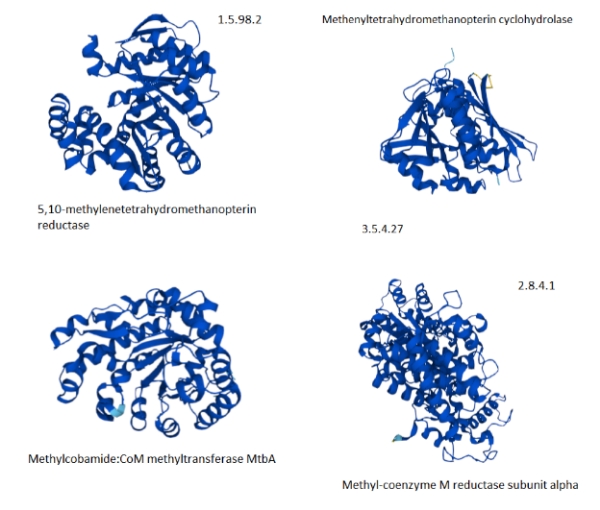
Figure 7. Selected proteins from the methane metabolism pathway of archaea showing the predominance of helix structures [The Kegg #.#.#.# identifiers are shown to map to figure 5.].
The paucity of good, available tertiary protein structures for the methanogenesis pathways makes the selection support a more anecdotal than analytic explanation. The selected proteins do suggest that they are highly composed of alpha-helices. If the methanogenesis pathways are more highly populated with proteins with helical structures, then the explanation of the hot vent-living archaeans might hold.
In other words, it is not, particularly the energetic favorability that determines the proteome composition, but rather the types of metabolic pathways, most likely methanogenesis that is responsible. It should be noted that pathway proteins are not populated by one unique protein as the Kegg pathway indicates where several closely related genes/proteins can be involved in the same function.
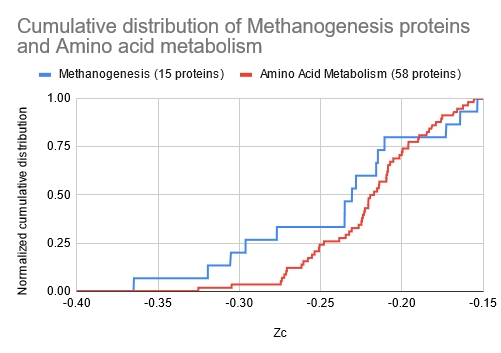
Figure 8. Cumulative distribution of proteins for methanogenesis and amino acid metabolism for M. jannaschii. The methanogenesis proteins are biased towards the lower Zc values, indicating a greater probability of alpha-helix structures.
Figure 7 shows the normalized cumulative distributions of 15 methanogenesis proteins and 58 amino acid metabolizing proteins that have been well identified for M. jannashii.
The distribution clearly shows that there is a bias towards the lower Zc values for the methanogenesis proteins than the more widely distributed amino acid metabolic proteins. While not definitive, it is suggestive that the proteome Zc score distribution between organisms may be accounted for by the presence and numbers of methanogenesis proteins.
Lastly, I want to touch on some speculation on the larger question of abiogenesis. It is unclear whether bacteria or archaea are the older life forms and closer to the last universal common ancestor (LUCA). Because the archaea share some similarities to the eukaryotes, this implies that either the bacteria are the earlier form, or that they are a later form that branched off from the archaea, and the eukaryotes evolved from the archaean branch. The attractiveness of the archaea as the most ancestral forms, as their domain name suggests, is their extremophile nature and their ability to extract energy from the geologic production of H2 to form CH4 as autotrophs, rather than consuming CH4, which has been shown to be relatively out of equilibrium due to the energy barrier to complete the reaction.
If so, does the energetic favorability of amino acid formation at ultramafic hot vent locations suggest a possible route to abiogenesis via a metabolism first model? While the reaction to create amino acids abiotically may be difficult to proceed, they may accumulate over time as long as the reverse reactions to degrade them are largely absent. As peptide bonds are energetically favored, oligopeptides and proteins could form abiotically at the vents as the hot fluids mix with the cold ocean water.
If so, could random small proteins form autocatalytic sets that lead to metabolism and reproduction? A number of experiments indicate that amino acids will spontaneously link together and that they can be autocatalytic for self-replication. Peptides replacing the sugar-phosphate backbone can link nucleobases that also can replicate, the model that was held to be a feature of the RNA World model.
But there is a potential fly in the ointment of this explanation of abiogenic protein formation. The proteins should be formed from amino acids that are composed of both L and D chiral forms. Life has selected one form and is homochiral, a feature that is suggested as a determinant for the origin of any extraterrestrial biologically important molecules detected. Experiments have suggested that any small bias in chirality, due perhaps to the crystal surface structure of the rocks, can lead to an exponential dominance of one chiral form over the other. Ribo et al published a review of this spontaneous mirror symmetry breaking (SMSB) [5].
So we have a possible model of abiotically formed peptides of random amino acid sequences that collect in the pores of rocks at the vents and may be surrounded by lipid membranes. The proteins can both form metabolic pathways and self replicate. If the peptides mostly form self-replicating helices, and these can be co-opted to further extract energy via methanogenesis, then we have a possible model for the emergence of life.
As my earlier article speculated that radiolysis could ensure that chemotrophs in the crust of a wide variety of planets and moons could be supported, we can now speculate that the favorable energetics of amino acid and protein formation may also drive the emergence of life.
As autotrophic organisms like archaea can evolve to exploit the energetics of CH4 and protein production under favorable conditions at seafloor vents, and support the evolving ecosystems of chemotrophs, this suggests that abiotic reactions may have started the process that evolved into the sophisticated methanogenesis pathways of methanogens we see today.
If correct, then life may be common in the galaxy wherever the conditions are right, that is that where ultramafic rocks in the mantle, heated from below by various means, and in contact with cold ocean water exist in combination, whether on a planet similar to the early Earth or possibly at the boundary of the mantle and the deep subsurface oceans of icy moons outside the bounds of the traditional habitable zone.
References
Tolley, A “Radiolytic H2: Powering Subsurface Biospheres” (2021) URL accessed 12/01/2021:
https://www.centauri-dreams.org/2021/07/02/radiolytic-h2-powering-subsurface-biospheres/
Dick, J, Shock, E. “The Release of Energy During Protein Synthesis at Ultramafic-Hosted Submarine Hydrothermal Ecosystems” (2021) JournalJournal of Geophysical Research: Biogeosciences, v126:11.
https://agupubs.onlinelibrary.wiley.com/doi/full/10.1029/2021JG006436
Uniprot database
uniprot.org
Kegg database
genome.jp/kegg/
Ribo, J et al “Spontaneous mirror symmetry breaking and origin of biological homochirality” (2017) Journal of the Royal Society Interface, v14:137
https://royalsocietypublishing.org/doi/10.1098/rsif.2017.0699
Alpha-Helix
https://en.wikipedia.org/wiki/Alpha_helix